New “Prime Editing” Method Makes Only Single-Stranded DNA Cuts
A new gene editing technique called prime editing, tested in human and mouse cells, rewrites DNA by only cutting a single strand to add, remove, or replace base pairs. The method may allow researchers to edit more types of genetic mutations than existing genome-editing approaches such as CRISPR-Cas9, researchers report today (October 21) in Nature.
Emma Haapaniemi, a group leader at the Center for Molecular Medicine Norway who studies gene editing to treat rare diseases and wasn’t part of the work to develop prime editing, tells The Scientist that the approach is “innovative and novel,” though of course, the technique is "still a prototype" and will need to be refined.
The discovery that CRISPR-Cas9 could be harnessed and used to edit animal and human genes ushered in a new era of genetic research over the past several years. The technique has become an indispensable tool in many research laboratories, allowing scientists to more easily create animal models of genetic diseases. While CRISPR-Cas9 has been making its way toward the clinic, its practical use in curing human disease has been limited by ethical considerations, challenges with delivery, and precision—in particular, so-called off-target effects that alter DNA at unintended loci in the genome.
The CRISPR-Cas9 gene editing system has been known to produce extra cuts in wrong sections of DNA, which can interrupt cell function. Another type of gene editing that doesn’t rely on DNA breaks and was thought to minimize inaccuracy is base editing, in which an enzyme can trade one DNA nuclease for another, but this strategy offers limited options as it can only make four of the 12 possible base pair changes, and some recent work has suggested it’s not as precise as scientists first thought.
When postdoc and lead author of the study Andrew Anzalone joined David Liu’s lab at the Broad Institute, which previously developed the technique for base editing, he was especially excited by the possibility of editing genes without using DNA breaks.
A new gene editing technique called prime editing, tested in human and mouse cells, rewrites DNA by only cutting a single strand to add, remove, or replace base pairs. The method may allow researchers to edit more types of genetic mutations than existing genome-editing approaches such as CRISPR-Cas9, researchers report today (October 21) in Nature.
Emma Haapaniemi, a group leader at the Center for Molecular Medicine Norway who studies gene editing to treat rare diseases and wasn’t part of the work to develop prime editing, tells The Scientist that the approach is “innovative and novel,” though of course, the technique is "still a prototype" and will need to be refined.
The discovery that CRISPR-Cas9 could be harnessed and used to edit animal and human genes ushered in a new era of genetic research over the past several years. The technique has become an indispensable tool in many research laboratories, allowing scientists to more easily create animal models of genetic diseases. While CRISPR-Cas9 has been making its way toward the clinic, its practical use in curing human disease has been limited by ethical considerations, challenges with delivery, and precision—in particular, so-called off-target effects that alter DNA at unintended loci in the genome.
The CRISPR-Cas9 gene editing system has been known to produce extra cuts in wrong sections of DNA, which can interrupt cell function. Another type of gene editing that doesn’t rely on DNA breaks and was thought to minimize inaccuracy is base editing, in which an enzyme can trade one DNA nuclease for another, but this strategy offers limited options as it can only make four of the 12 possible base pair changes, and some recent work has suggested it’s not as precise as scientists first thought.
When postdoc and lead author of the study Andrew Anzalone joined David Liu’s lab at the Broad Institute, which previously developed the technique for base editing, he was especially excited by the possibility of editing genes without using DNA breaks.
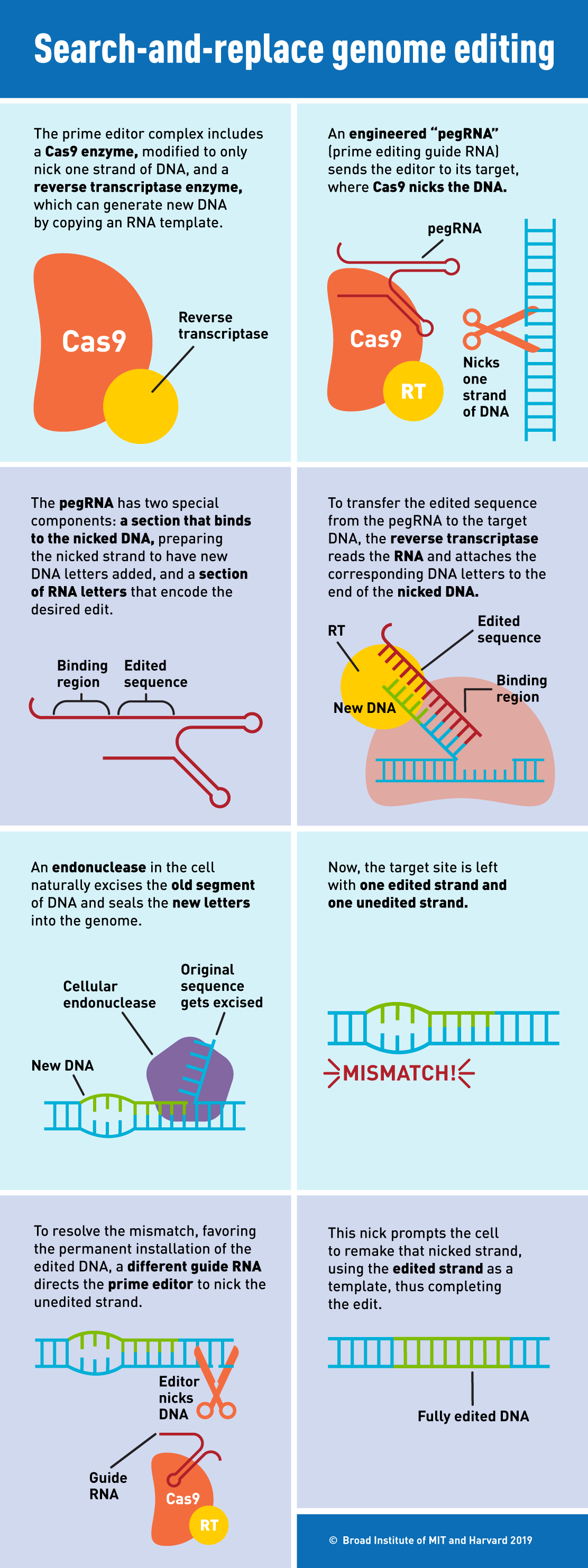
So building on what the two knew about base editing and CRISPR-Cas9, they began working on a new technique to cut just one strand of DNA, leaving the other intact. It uses the same Cas9 nuclease as frequently deployed in the CRISPR system but combines the enzyme with two new reagents: a guide RNA called pegRNA, which leads Cas9 to the desired spot in the genome, and a reverse transcriptase that initiates the addition of a new sequence or base into the genome. Once the new genetic material is incorporated into the cut strand of DNA, the prime editor nicks the unedited strand, signaling to the cell to rebuild it to match the edited strand.
The team tested prime editing in vitro in four different types of human cell and mouse neurons.
“What we observed was pretty remarkable,” Liu said in a press conference. In order to compare its accuracy to CRISPR-Cas9, the team used its technology to edit four genetic mutations. Previous studies had shown that when CRISPR-Cas9 targeted these same mutations, it caused off-target DNA changes in 16 predictable locations. Prime editing only altered three of these loci, suggesting it is more precise than CRISPR-Cas9.
Prime editing is more complex than CRISPR editing. It requires three separate steps in which the DNA must match up with parts of the prime editing system. Liu says he believes this might be the secret to its accuracy. CRISPR-Cas9 relies on one pairing step: the guide RNA must pair with the target DNA. Prime editing requires this first step, but also includes two more components, a part of the guide RNA called the primer must also bind to the target site and the newly introduced DNA must bind to the original site. “If any one of those three DNA pairing events fail, then prime editing can’t proceed,” says Liu. “We believe that those three independent pairing events each provide an opportunity to reject off-target sequences,” he adds.
Nicholas Katsanis, who studies genetic medicine at the Children’s Hospital of Chicago, says that “there’s no denying that there’s some off-target effect [with CRISPR-Cas9] but the fear of the off-target effects is more than the reality.” Nonetheless, he’s excited by the new tool and "enamored" of some aspects of the new technology, such as its potential ability to edit a greater diversity of targets than other gene editing methods can.
Liu and his team used prime editing to target genes underlying Tay-Sachs disease and sickle cell anemia. The mutations were changed back to healthy DNA sequences with 35–55 percent efficiency, similar to rates that would likely be achieved with CRISPR-Cas9 editing.
The Broad Institute has licensed the technique to Prime Medicine, a company cofounded by Liu, under the institute’s "inclusive innovation" model, which allows Prime to exclusively use the technology to aim at certain targets, but offers other companies the opportunity to apply to license it for editing other genes. Liu’s group has also made the technique available on the Addgene database for academic use. “I’m hopeful that hundreds if not thousands of researchers will try to use prime editing in the coming weeks and months and years,” says Liu. “It’s really important that the community test and, if needed, optimize prime editing in as many different types of cells and organisms as possible.”
A. Anzalone et al, “Search-and-replace genome editing without double-strand breaks or donor DNA,” Nature, doi:10.1038/s41586-019-1711-4, 2019.
Keywords: base editingCas9CRISPRgene editinggenetics & genomicsNewsprime editingtechniques
Ubigene Biosciences is co-founded by biological academics and elites from China, the United States, and France. We are located in Guangzhou Science City, which serves as a global center for high technology and innovation. Ubigene Biosciences has 1000㎡ office areas and laboratories, involving genome editing, cell biology technology, and zebrafish research. We provide products and services for plasmids, viruses, cells, and zebrafish. We aim to provide customers with better gene-editing tools for cell or animal research.
We developed CRISPR-U™ and CRISPR-B™(based on CRISPR/Cas9 technology) which is more efficient than general CRISPR/Cas9 in double-strand breaking, CRISPR-U™ and CRISPR-B™ can greatly improve the efficiency of homologous recombination, easily achieve knockout (KO), point mutation (PM) and knockin (KI) in vitro and in vivo.
Genome Editing Platform
——Focusing on the Application of CRISPR-U™ and CRISPR-B™ Gene Editing Technology
Cell Biology Platform
——Focusing on primary cell
2. Provides culture strategies and related products for different cell types.3. Provides cell biology-related services such as cell isolation, extraction and validation.