Practical Hep 3B Cell Culture and Gene-Editing Protocols

Content

Expert Insights | Practical Hep 3B Cell Culture and Gene-Editing Protocols
Hep 3B cells are a widely used model in hepatocellular carcinoma (HCC) research. Derived from human liver cancer tissue, these cells exhibit stable phenotypic characteristics and partially recapitulate the in vivo metabolic behavior of hepatoma cells. With robust proliferative capacity and high experimental reproducibility, Hep 3B cells are commonly applied in studies of hepatocellular carcinoma biology, anti-tumor drug screening, and functional gene validation. Morphologically, Hep 3B cells resemble HepG2 cells; however, a key distinction is that Hep 3B cells harbor an integrated and transcriptionally active hepatitis B virus (HBV) genome, making them particularly valuable for investigating HBV infection and its oncogenic progression.To fully harness the potential of Hep 3B cells in experimental research, meticulous attention to culture and handling practices is essential to minimize variability and ensure reliable data output. Today, Ubigene is sharing its expert insights — the ultimate guide to optimizing Hep 3B cell culture and gene-editing techniques — to help you achieve consistent and high-quality results.
I. Overview of Human Hepatoma Cell Line (Hep 3B)
Cell Name: Human Hepatoma Cell Line(Hep 3B)
Morphology: Epithelial-like, adherent growth
Culture Medium: 87% MEM + 10% FBS + 1% GlutaMAX + 1% Sodium Pyruvate + 1% NEAA
Gas Phase: 95% air, 5% CO₂
Temperature: 37 °C
Medium Change Frequency: Replace culture medium every 2–3 days
Subculture Ratio: 1:2 – 1:4
Reference for Hep 3B Cell Growth Status
● Normal status: Cells display a polygonal, epithelial-like shape with clearly defined cell–cell boundaries under the microscope. They grow as a non-overlapping monolayer with a full and refractive cytoplasm (as shown below).
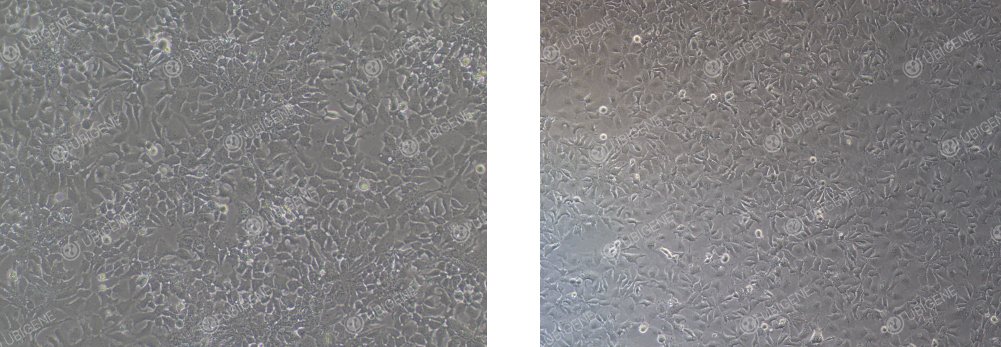
● Abnormal morphology: Cells become elongated and spindle-shaped, appear flattened with poor refractivity, and exhibit cytoplasmic vacuolation or increased cellular debris, indicating stress or suboptimal culture conditions (as shown below).
II. Hep 3B Cell Thawing Procedure
1. Preparation of Complete Medium
Pipette 7 mL of complete medium into a sterile centrifuge tube and keep it ready for use.
2. Cell Thawing
Retrieve the frozen Hep 3B cell vial from dry ice. Using sterilized forceps, hold the cap and gently swirl the vial in a 37 °C water bath (do not submerge the cap). Thaw rapidly until only a few small ice crystals remain — typically within ~1 minute. Immediately remove from the water bath.
3. Centrifugation
Transfer the thawed cell suspension into the prepared centrifuge tube. Centrifuge at 1100 rpm for 4 minutes to pellet the cells. Carefully discard the supernatant to remove residual cryoprotectant (DMSO).
4. Resuspension and Seeding
Gently resuspend the cell pellet in fresh complete medium and seed into an appropriately sized culture dish or flask, ensuring even distribution of cells.
5. Cell Culture
Place the culture vessel in a 37 °C incubator with 5% CO₂. After 24 hours, observe cell attachment and morphology under the microscope to confirm recovery and adherence quality.
III. Hep 3B Cell Passaging (e.g., T25 Flask)
1. Subculture Conditions
● Passage the cells when they reach 80–90% confluence.
● Passage the cells when they reach 80–90% confluence.
● In a biosafety cabinet, carefully aspirate the spent medium and wash the cell layer 1–2 times with 5 mL PBS to remove serum residues that may inhibit trypsin activity.
2. Trypsinization
● Add 1 mL of trypsin–EDTA solution to the flask, gently tilt and rotate to ensure the entire surface is covered.
● Incubate at 37 °C for 2–3 minutes.
● Under the microscope, monitor the cells — once most have rounded up and detach easily with gentle tapping, immediately proceed to neutralization.
3. Neutralization of Trypsin
● Add 2 mL of complete medium (twice the volume of trypsin) to stop digestion.
● Gently pipette to obtain a single-cell suspension and transfer the mixture into a 15 mL centrifuge tube.
4. Centrifugation
● Centrifuge at 1100 rpm for 4 minutes at room temperature.
● Discard the supernatant and gently resuspend the cell pellet in fresh complete medium.
5. Cell Passaging
● Inoculate the cells into new culture flasks at a 1:2 to 1:4 split ratio, depending on growth density.
● Place the flasks in a 37 °C, 5% CO₂ incubator, and check cell morphology and attachment the following day to ensure optimal recovery.
IV. Hep 3B Cell Cryopreservation Procedure
1. Cell Collection
Harvest cells following the standard passaging procedure and transfer the trypsinized single-cell suspension into a centrifuge tube.
2. Centrifugation
Centrifuge at 1100 rpm for 4 minutes at room temperature and carefully discard the supernatant.
3. Resuspension and Aliquoting
● Gently resuspend the cell pellet in cryopreservation medium, ensuring a final concentration of 1 × 10⁶ cells/mL.
● Aliquot 1 mL per cryovial, and clearly label each vial with cell line name, passage number, and date.
4. Controlled Freezing and Storage
Place the vials in a programmed-rate freezing container and store at −80 °C overnight to achieve gradual cooling.The next day, transfer the vials to liquid nitrogen for long-term storage.
V. Hep 3B Cell Culture Considerations
1. Medium and Serum: Use the correct basal medium with the appropriate serum concentration.Store prepared complete medium at 4 °C and use within 2 weeks for optimal performance.
2. Culture Environment: Ensure that incubator conditions (temperature, CO₂ level, humidity) are stable and within specifications.
3. Pre-warming Reagents: Pre-warm culture medium and trypsin to 37 °C before use to avoid temperature shock to the cells.
4. Avoid Over-digestion: Hep 3B cells are sensitive to enzymatic digestion; carefully monitor digestion time and trypsin concentration to prevent cell damage.
5. Subculture Handling: Minimize vigorous pipetting to avoid damaging cell membranes.If cells are unevenly attached, gently shake the flask to distribute them evenly.
6. Black Specks in Background: Dark particles may appear in the medium due to metabolic byproducts or cell debris; these do not affect cell growth.If excessive, gently rinse the cells 1–2 times with PBS during medium change to reduce debris.
VI. Hep 3B Cell Transfection Considerations
1. Cell condition requirements
● Use cells in logarithmic growth phase with 70–80% confluence.
● Ensure cell viability >85%, as determined by Trypan Blue exclusion.
● Prefer low-passage cells
● Carefully monitor enzymatic digestion to prevent over-trypsinization and cell damage.
● Maintain a single-cell suspension to avoid clumping during transfection.
2. Transfection Reagents and Pre-testing
● Thoroughly mix transfection reagents before use to ensure uniformity.
● Conduct preliminary screening experiments to determine optimal selection drug concentrations for post-transfection selection.
3. Electroporation
● Control the number of cells; seed cells into appropriate plates post-electroporation.
● Use mild trypsinization and neutralize with serum-containing medium.
● Wash cells 1–2 times with PBS to remove residual serum and ions that may interfere with electroporation.
● Optimize electroporation parameters through pre-experiments.
● Ensure post-electroporation attachment rate ≥70%.
● Keep total electroporation time short to minimize stress.
4. Lentiviral Transduction
● Determine the optimal MOI through pre-testing.
● Maintain 30–40% cell confluence at the time of infection.
● Add Polybrene to enhance viral entry.
● Replace medium 24 h post-infection.
● Avoid repeated freeze-thaw cycles of viral stocks.
● For low transduction efficiency, consider re-infection (ensuring cells tolerate virus well) or spinoculation.
5. Lipid-mediated Transfection
● Choose reagents compatible with Hep 3B cells.
● Optimize DNA-to-reagent ratio in pre-experiments to determine the best transfection conditions.
● Use any enhancers provided in the system to improve efficiency for difficult-to-transfect cells.
● Plate cells 24 h prior to transfection; target 60–70% confluence at the time of transfection.
● Dilute DNA in Opti-MEM first, then add lipid reagent (reverse addition can reduce efficiency).
● Allow complexes to incubate at room temperature for 15–20 min (too short: incomplete complexing; too long: increased cytotoxicity).
● Do not add antibiotics to transfection medium; cationic lipids increase cell permeability and antibiotics may cause toxicity.
● Add complexes slowly and evenly to the medium, gently swirling to mix; avoid direct impact or vigorous pipetting that can damage cells.
VII. Hep 3B Single-Cell Cloning (Clone Screening) Considerations
1. Cell condition
● Use cells in the logarithmic growth phase; target ~70% confluence before seeding for single-cell cloning.
● Ensure cell viability ≥85% at the time of cloning.
2. Reagents and Pre-testing
● Pre-warm all reagents, including culture medium, trypsin, and PBS, to 37 °C before use.
● Prefer gentle dissociation reagents (e.g., TrypLE Express) to minimize cell stress.
3. Plating Strategy
● Conduct preliminary experiments to determine the optimal seeding density, avoiding too low a single-cell occupancy.
● When seeding into 96-well plates, ensure even cell distribution. Fill outer wells with PBS to reduce evaporation and maintain uniform conditions.
4. Dilution Method
● Use the limiting dilution method for single-cell seeding.
● After counting, aim for a final cell concentration of 1 × 10⁶ – 2 × 10⁶ cells/mL to achieve efficient single-cell growth.
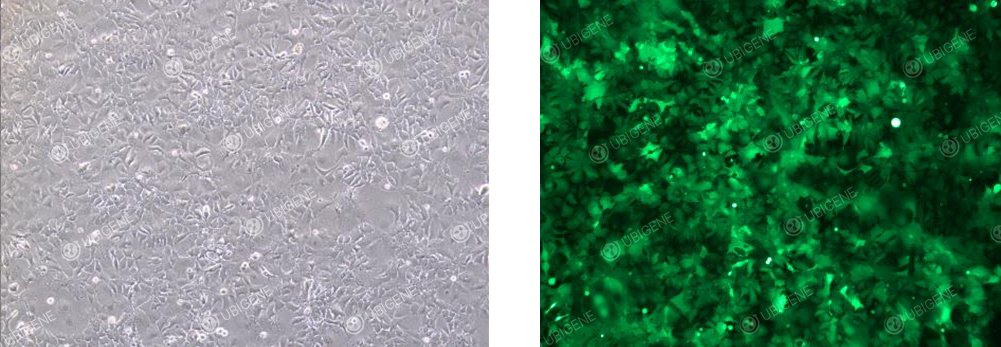
Cell Images of Cells Following Lentiviral Transduction
VIII. Hep 3B Cell Culture Common Issues and Solutions
1. Slow or Stalled Cell Growth
Observation: Cells divide slowly, fail to reach confluence in expected time; medium color changes slowly.
Possible Causes:
(1) Serum issues: Poor quality, low concentration, or batch inconsistency.
(2) Cell-related issues: Low seeding density during passaging; aged cells with high passage number.
(3) Environmental issues: Incubator temperature or CO₂ levels inaccurate or unstable.
(4) Nutrient depletion: Infrequent medium changes leading to metabolite accumulation.
Solutions:
(1) Use high-quality, pre-tested fetal bovine serum (FBS); temporarily increase concentration to 15% if needed.
(2) Ensure cells are 80–90% confluent at passaging and seed sufficient cell numbers.
(3) Regularly calibrate incubators to maintain stable environmental conditions.
(4) Change medium every 2–3 days to maintain nutrient availability.
2. Altered Cell Morphology
Observation: Elongation/fibroblast-like: Polygonal cells become spindle-shaped. Vacuolation: Large transparent vacuoles appear in the cytoplasm. Increased granularity: Cytoplasm contains numerous dark particles.
Possible Causes:
(1) Poor serum quality.
(2) Nutritional stress: insufficient feeding or infrequent medium changes.
(3) Toxic stress: drug treatment or accumulation of waste metabolites
(4) Cell aging or spontaneous differentiation.
Solutions:
(1) Replace with high-quality serum.
(2) Increase feeding frequency to ensure sufficient nutrients.
(3) Check the system for toxic compounds or contaminants.
(4) Use low-passage cells or perform clonal selection to restore typical morphology.
3. Rapid Medium Acidification (Yellowing)
Observation: Medium turns yellow within hours or overnight after a change.
Possible Causes:
(1) High cell density: Excessive metabolic activity.
(2) Low CO₂ concentration: Medium becomes slightly alkaline initially; cell metabolism acidifies it quickly.
(3) Abnormal cell metabolism: Metabolic byproducts may accumulate, leading to acidification.
Solutions:
(1) Passage cells before reaching 100% confluence.
(2) Verify and calibrate incubator CO₂ supply.
(3) Increase medium change frequency to maintain pH balance.
Pro Tip: Hep 3B cells secrete HBV-related antigens; during culture, it is recommended to change the medium regularly and avoid prolonged sealed incubation to prevent accumulation of metabolic byproducts that may compromise cell viability and function.
If you’re planning to use Hep 3B cells for hepatocellular carcinoma research, Ubigene’s Red cotton OmniCell bank offers over 1,000 wild-type cell lines, Click here to find your target cell line>>



