Overview
Ubigene provides a one-stop service for CRISPR/Cas9 vector construction and lentiviral packaging, meeting diverse gene editing needs for scientific research.The CRISPR/Cas9 system originates from a bacterial adaptive immune defense mechanism. It employs a sequence-specific guide RNA (gRNA) to recognize and bind to a target DNA sequence, directing the Cas9 endonuclease to introduce a double-strand break (DSB) at the desired locus. The DSB is subsequently repaired by either non-homologous end joining (NHEJ) or homology-directed repair (HDR) pathways, enabling gene knockout, knock-in, or targeted mutation.The lentiviral vector, based on a pseudotyped HIV-1 system, is typically enveloped with VSVG to efficiently transduce both dividing and non-dividing cells. After infection, the exogenous RNA is reverse-transcribed and stably integrated into the host genome, resulting in long-term and stable gene expression with low immunogenicity. This makes lentiviral delivery highly suitable for in vitro cell experiments and in vivo animal studies.
View Picture
Technical schematic diagram

CRISPR/Cas9 Plasmid Construction
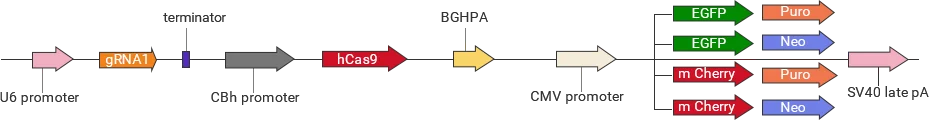
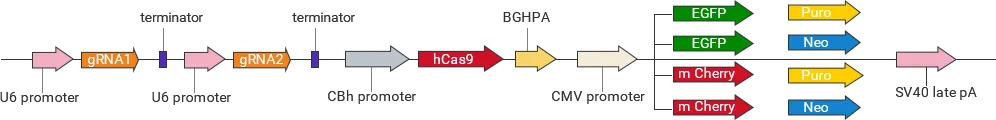
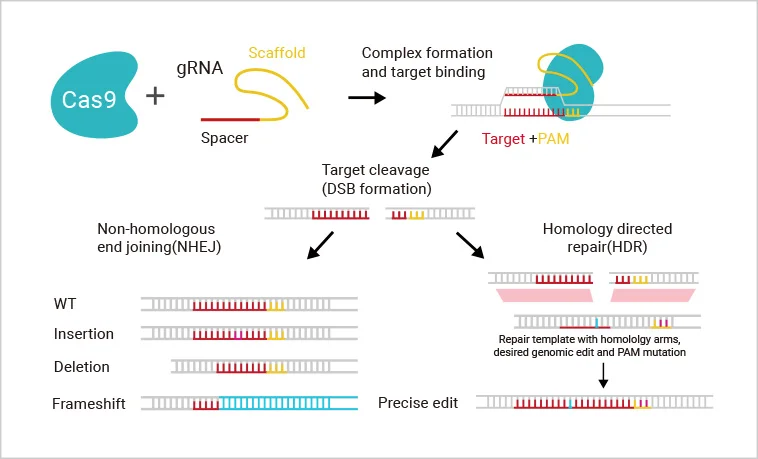
For each target gene, 2-3 guide RNAs (gRNAs) are designed to direct Cas9 to specifically recognize and cleave the target sequence upstream of the PAM site, thereby inducing a double-strand break (DSB) in the genomic DNA. The cell subsequently repairs the break through either non-homologous end joining (NHEJ) or homology-directed repair (HDR) . These repair processes can result in frameshift mutations or precise insertion of exogenous sequences, enabling gene knockout, knock-in, or point mutation.
Chat with an Expert
Lentiviral Transduction
The lentiviral vector is engineered by removing all pathogenic genes and inserting the gene of interest into the viral genome. Through in vitro packaging using a third-generation lentiviral system, high-titer viral particles are produced. Upon infection of host cells, the lentiviral RNA is reverse-transcribed into double-stranded DNA by reverse transcriptase and subsequently integrated into the host chromosome, enabling long-term and stable expression of the transgene.Due to its broad tropism, lentivirus can efficiently infect both dividing and non-dividing cells. In addition, its low immunogenicity minimizes host immune responses, making it an ideal tool for gene knockout/knock-in, overexpression, RNA interference (RNAi), microRNA studies, and in vivo gene therapy research.
Chat with an ExpertService Detail
Knockout Plasmid
Strategy Design:
Based on the customer's specific research requirements and the characteristics of the target gene, Ubigene provides customized gene knockout design solutions. The proprietary YKO series knockout vectors developed by Ubigene — including both lentiviral and conventional plasmid vectors — are versatile tools suitable for a wide range of in vitro and in vivo studies.
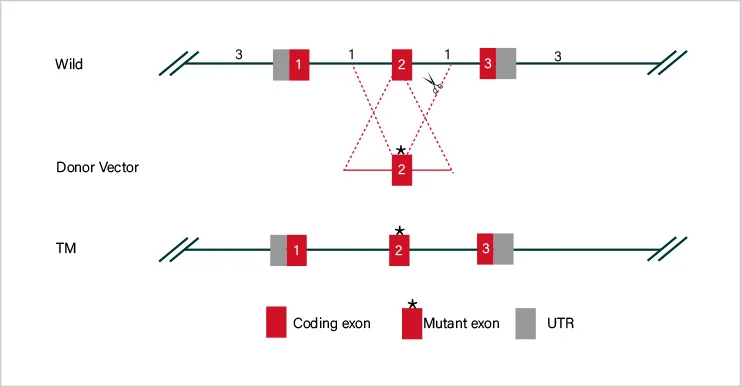
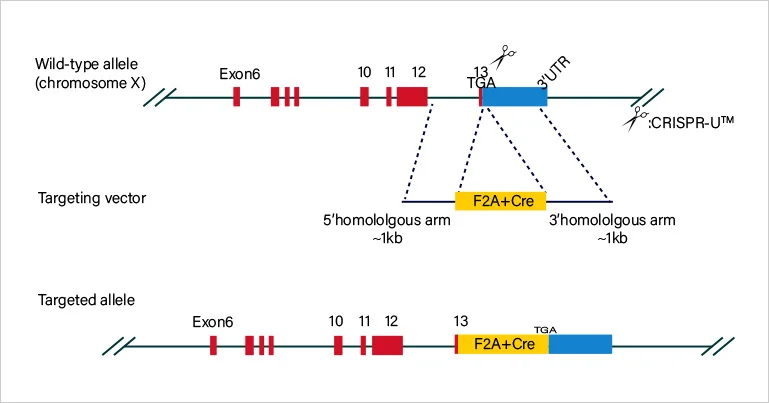
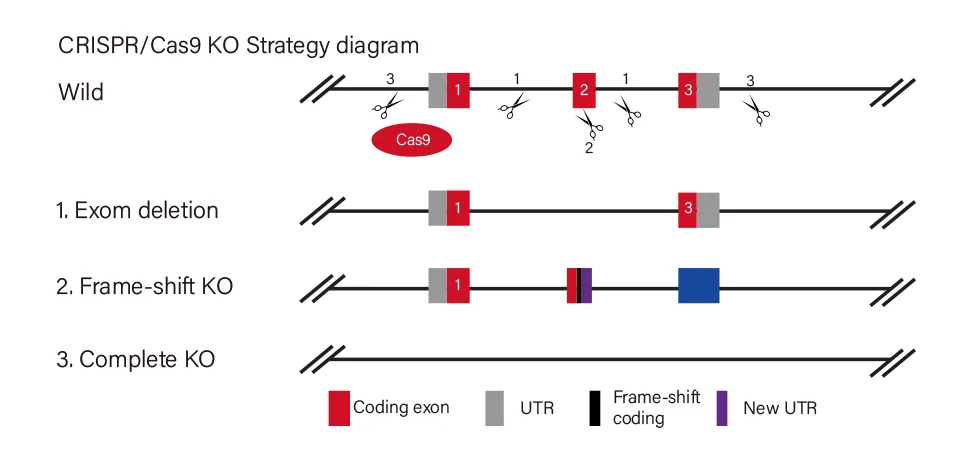
Short fragment removal
Guide RNAs target introns at both sides of exon 2 and the number of bases in exon 2 is not a multiple of 3, which can cause frame-shift mutation.
01
Frame-shift mutation
Guide RNA targets the exon, and the base number of deletion is not a multiple of 3. After knockout, frame-shift mutation would cause gene knockout.
02
Large fragment removal
Complete removal of the coding sequence to achieve gene knockout.
03
View Picture
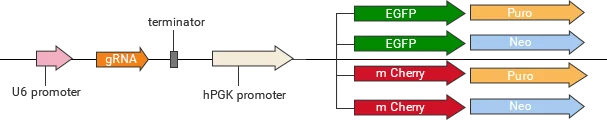
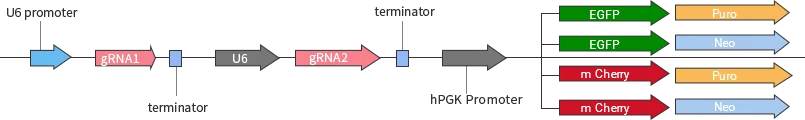
Classification of knockout gRNA plasmids
Ubigene developed a series of YKO plasmids, including lentivirus plasmids, AAV plasmids, and non-viral plasmids, which can be widely used in vitro or in vivo.
| YKO series | Vector | Reporter gene; Selection marker |
|---|---|---|
| Lentiviral YKO plasmid | YKO-LV001-single-gRNA | EGFP/mCherry, Puro/Neo |
| YKO-LV001-dual-gRNA | EGFP/mCherry, Puro/Neo | |
| Non-viral YKO plasmid | YKO-RP001-single-gRNA | EGFP/mCherry, Puro/Neo |
| YKO-RP001-dual-gRNA | EGFP/mCherry, Puro/Neo | |
| AAV YKO plasmid | YKO-AAV001-single-gRNA | EGFP/mCherry |
| YKO-AAV001-dual-gRNA | EGFP/mCherry |
Quality Control
Comprehensive quality control is implemented throughout the vector construction process. Restriction enzyme digestion and Sanger sequencing validation reports are provided to ensure the accuracy and integrity of the constructs.
Application
Ubigene's gene editing vectors are designed for a broad range of research applications, targeting diverse genetic modification needs across different cell lines as well as murine models (mice and rats). These vectors are suitable for various experimental purposes, including gene knockout, knock-in, mutation, and functional studies.
Workflow
Plasmid restriction enzyme
digestion and sequencing report
Plasmid construction

Maxi prep

AAV packaging

Transduction validation

qPCR confirm titer
Plasmid construction
Maxi prep
AAV packaging
Transduction validation
qPCR confirm titer
FAQs
1. How long does it take for gene expression after transduction?
It takes a long time for expression after lentivirus transduction. But fluorescence can be observed 24 hours after transduction on the cells with strong metabolism (such as 293T, HEK293, etc.); the fluorescent protein expression of cells with slow metabolism (such as primary culture cells, neural stem cells, embryonic stem cells, etc.) takes a longer time, i.e. 72-96 hours or even longer after transduction. The transduced cells can be continuously cultured for one week, and the effect of transduction of the virus to the target cells can be determined by observing the expression time and intensity of fluorescence.
2. How to improve transduction efficiency?
Ensure that the cells are in good growth condition before transduction. Use 293T for titer test to verify whether the titer of virus drops significantly; properly increase the MOI and extend the transduction time.
3. After lentivirus transduction, the condition of cells is very poor or even a large number of deaths. How to solve this problem?
Poor cell conditions before transduction, exceeding MOI, and cytotoxicity of target genes may lead to abnormal cell conditions.The main solutions are:
- Make sure the pre-transduction cell condition;
- Reduce the MOI to verify the cytotoxicity of the target gene;
- Observe the cells after 6 hours of transduction, change the fresh medium in case of abnormality of cells, if not, change the medium 24 hours later.