Expert Insights | Practical Tips for ARPE-19 Cell Culture and Gene Editing


ARPE-19 cells are derived from the retinal pigment epithelium of a healthy 19-year-old male who died from a head injury in a motor vehicle accident. This cell line retains key functions such as pigment synthesis and phagocytosis of photoreceptor outer segments. It also demonstrates stable in vitro growth and efficient passaging, making it a widely used model for studying retinal degenerative disease mechanisms, intraocular inflammation regulation, ocular drug screening, and retinal repair. ARPE-19 is thus an indispensable classic cell model in both basic and translational ophthalmic research. Here, we share exclusive ARPE-19 culture tips, offering a comprehensive guide to mastering both the optimal culture techniques and practical gene-editing strategies for this cell line.
Overview of Human Retinal Pigment Epithelial Cell Line (ARPE-19)
- Cell Name: Human Retinal Pigment Epithelial Cell Line (ARPE-19)
- Cell Morphology: Epithelial-like; adherent growth
- Culture Medium: 90%DMEM/F12+10%FBS
- Atmosphere: 95% air, 5% CO₂
- Temperature: 37℃
- Medium Renewal Frequency: Every 2-3 days
- Passage Ratio: 1:3-1:4
Reference for Cell Growth Status
- Normal Morphology: Cells exhibit a polygonal to irregular polygonal shape. They form a tightly connected monolayer with a cobblestone-like arrangement. Cell organization is orderly, with clearly defined boundaries.
- Abnormal Morphology: Cells show enlarged and elongated bodies, becoming irregular in shape. Some cells shrink and round up, exhibiting poor adherence. Increased cell debris or transparent cytoplasmic vacuoles may be observed.
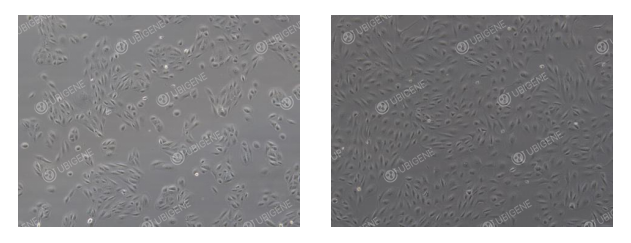

ARPE-19 Cell Thawing Procedure
-
Prepare Culture Medium
Add 7 mL of complete culture medium to a centrifuge tube and keep it ready. -
Thaw Cells
Remove the vial from dry ice. Hold the cap with forceps and place the vial in a 37°C water bath. Gently swirl the vial (ensure water does not cover the cap) until the cells are nearly thawed—ice should reduce to the size of mung beans, approximately 1 minute. Then remove from the water bath. -
Centrifuge Cells
Transfer the thawed cell suspension to a centrifuge tube. Centrifuge at 1100 rpm for 4 minutes and discard the supernatant. -
Resuspend and Seed Cells
Resuspend the cell pellet in complete culture medium and seed into an appropriately sized culture dish or flask. -
Culture Cells
Place the culture dish or flask in a 37°C incubator. After 24 hours, observe cell attachment and morphology.
ARPE-19 Cell Subculture Procedure (e.g., T25 Flask)
1. Subculture Conditions
- Subculture cells when they reach 80–90% confluency.
- In a biosafety cabinet, discard the culture medium and wash the cells 1–2 times with 5 mL PBS.
2. Trypsin Digestion
- Add 1 mL of trypsin, gently swirl the flask to ensure the enzyme fully covers the cells.
- Place the flask in a 37°C incubator for 2-3 minutes.
- Under a microscope, when most cells become round and bright, gently tap the sides of the flask to detach cells, and immediately stop the digestion.
3. Terminate Digestion
- Add 2 mL of complete culture medium (2* the volume of trypsin) to stop the reaction.
- Transfer the cell suspension to a 15 mL centrifuge tube.
4. Centrifuge Cell Suspension
- Centrifuge at 1100 rpm at room temperature for 4 minutes.
- Discard the supernatant and resuspend the cell pellet in complete culture medium.
5. Subculture and Culture
- Seed cells at a split ratio of 1:2-1:3.
- Observe cell morphology and attachment the next day.
ARPE-19 Cell Cryopreservation Procedure
-
Collect Cells
Harvest trypsinized cells following the standard subculture procedure and transfer them into a centrifuge tube. -
Centrifugation
Centrifuge at 1100 rpm for 4 minutes and discard the supernatant. -
Resuspend and Aliquot for Freezing
Resuspend the cell pellet in cryopreservation medium. Adjust the concentration to 1*10^6 cells/mL and aliquot 1 mL per cryovial. Label each vial with cell line name, passage number, and date. -
Cooling and Storage
Place the vials in a controlled-rate freezing container and store overnight at -80°C. Transfer the vials into liquid nitrogen for long-term storage.
ARPE-19 Cell Culture Notes
- Cell Confluency: Subculture cells at 80-90% confluency for optimal growth.
- Medium Change: Replace with fresh complete medium every 2-3 days for actively growing cultures.
- Medium Storage: Store culture medium at 4°C, protected from light, and use within the expiration date.
- Culture Environment: Maintain a stable and appropriate incubator environment.
- Operational Details: Pre-warm culture medium and trypsin to 37°C before use to avoid temperature stress.
- Subculture Handling: Avoid excessive pipetting to prevent cell membrane damage; gently swirl the flask if cells are unevenly attached to achieve uniform distribution.
Precautions for ARPE-19 Cell Transfection
1. Cell Condition Requirements
- Ensure cells are healthy and in the logarithmic growth phase, with 70-80% confluency.
- Cell viability should be >85%, which can be assessed using Trypan Blue exclusion.
- Use low-passage cells.
- Carefully monitor trypsinization time to avoid over-digestion and cell damage.
- During the procedure, generate a single-cell suspension and avoid cell clumping.
2. Transfection Reagents and Pre-Experiment
- Mix transfection reagents thoroughly before use to ensure uniformity.
- Perform preliminary drug-selection experiments to determine the optimal selection concentration post-transfection.
3. Electroporation
- Control the number of cells and seed them into appropriately sized culture plates after electroporation.
- Use gentle trypsin and terminate digestion completely with serum-containing medium.
- Wash cells 1–2 times with PBS to completely remove residual serum, preventing ionic interference with electroporation.
- Optimize electroporation parameters through preliminary experiments.
- Ensure post-electroporation cell attachment rate is ≥70%.
- Keep total electroporation time short to avoid excessive stress.
4. Lentiviral Transduction
- Perform preliminary experiments to determine the optimal MOI.
- Maintain cell confluency at 30-40% before infection.
- Add Polybrene prior to infection to enhance viral uptake.
- Change medium 24 hours after infection.
- Avoid repeated freeze-thaw cycles of viral stocks.
- If infection efficiency is low, consider repeat infection (ensuring cell tolerance) or use centrifugation-enhanced transduction.
5. Liposome-Based Transfection
- Choose an appropriate liposome reagent and optimize the DNA-to-reagent ratio through preliminary experiments.
- Use enhancer reagents provided by some transfection systems to improve efficiency, especially in hard-to-transfect cells.
- Prepare liposome-DNA complexes in serum-free medium (e.g., Opti-MEM), as serum can interfere with complex formation and reduce efficiency.
- Plate cells 24 hours before transfection, targeting 60–70% confluency at the time of transfection.
- Dilute DNA in Opti-MEM first, then add the liposome reagent (reverse mixing can reduce efficiency).
- Incubate the mixture at room temperature for 15-20 minutes (less than 10 minutes may result in incomplete complex formation; more than 30 minutes may increase cytotoxicity).
- Do not add antibiotics to the transfection medium, as cationic lipids increase cell permeability, potentially causing antibiotic toxicity.
- Add complexes slowly and evenly to the culture medium, gently swirling to mix; avoid directly pipetting onto cells or vigorous pipetting to prevent damage.
Precautions for ARPE-19 Single-cell cloning experiment
1. Cell Condition Requirements
- Use cells in the logarithmic growth phase for single-clone seeding. Before seeding, maintain approximately 70% confluency.
- Cell viability should be ≥80% at the time of seeding.
2. Reagents and Pre-Experiment Preparation
- Pre-warm all reagents, including culture medium, trypsin, and PBS, prior to the experiment.
- Use gentle dissociation reagents, such as TrypLE Express, to minimize cell damage.
3. Seeding Strategy
- Conduct preliminary experiments to determine the optimal seeding density gradient, avoiding too low a single-clone formation rate.
- When seeding 96-well plates, ensure even cell distribution. Fill the outer wells with PBS to prevent evaporation.
4. Dilution Method
- Employ the limiting dilution method for single-clone seeding.
- After cell counting and dilution, the optimal cell number should fall within 1 * 10⁶ to 2 * 10⁶ cells.
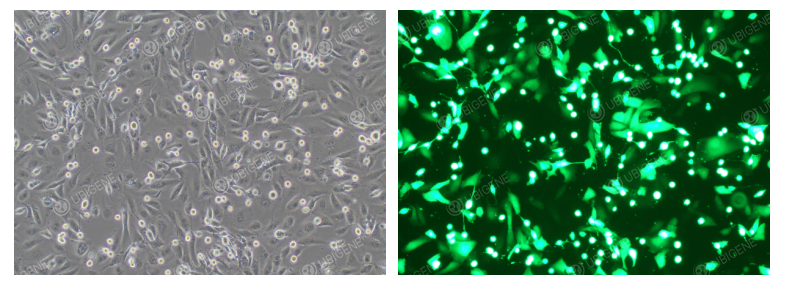
Cell Images After Electroporation
Common Issues in ARPE-19 Cell Culture and Solutions
1. Slow Cell Growth
Observation Cell proliferation is significantly slower than normal (normally passaged every 2-3 days).
Possible Causes
- Cell Senescence: Excessive passage number leading to reduced viability
- Low Seeding Density: Cells too sparse to establish an optimal growth environment.
- Suboptimal Culture Conditions: Incubator temperature, CO₂ concentration, or humidity are inaccurate or unstable.
- Mycoplasma Contamination: A common hidden cause of slow growth
- Unsuitable Culture Medium: Using medium not optimized for ARPE-19 (e.g., DMEM/F12) or low-quality serum (e.g., poor grade or batch variability).。
Solutions
- Control passage number to avoid over-passaging; subculture cells during logarithmic growth phase. Consider thawing early-passage frozen cells if needed.
- Adjust seeding density according to cell growth characteristics to an appropriate range.
- Verify incubator settings for temperature, CO₂ concentration, and humidity.
- Perform mycoplasma testing; if positive, discard the affected batch and thaw early-passage frozen cells, or use mycoplasma removal reagents (note: may alter cell properties).
- Use DMEM/F12 supplemented with 10% high-quality FBS; avoid frequently changing serum brands.
2. Abnormal Cell Morphology
Observation: Cells appear shrunken with unclear edges; enlarged, granular, vacuolated; epithelial-like cells transform into fibroblast-like morphology.
Possible Causes:
- Poor serum quality or cell senescence.
- Nutrient deficiency, over-digestion, or too low passage ratio.
- Accumulation of metabolic waste due to prolonged culture.
Solutions:
- Use validated, high-quality FBS; avoid unknown serum sources. Thaw early-passage frozen cells if necessary.
- Replace with fresh medium, adjust trypsinization time (generally 2-3 minutes), and use appropriate subculture ratios (e.g., 1:3 or 1:4) to ensure rapid monolayer formation after seeding.
- Perform timely subculture and medium changes; avoid waiting until 100% confluency. Optimal subculture is at 80-90% confluency.
If you are planning to use ARPE-19 cells for your research, we invite you to explore Ubigene Red Cotton OmniCell Bank. Ubigene offers over 1,000 wild-type cell lines spanning multiple research areas, providing comprehensive solutions to meet all your scientific needs.


