Ubigene
CRISPR Library In Vivo Screening
Recapitulating the native biological environment to drive scientific breakthroughs.
Why Choose CRISPR In Vivo Screening?
In basic research and drug development, CRISPR in vitro screening offers high throughput and flexibility, making it ideal for early-stage target discovery and mechanistic studies. However, to investigate complex biological questions—such as immune interactions, drug resistance mechanisms, tumor metastasis, and systemic effects—CRISPR in vitro screening alone is insufficient. CRISPR In vivo screening enables the study of gene function within the context of a complete organism, accurately recapitulating the native microenvironment and dynamic physiological conditions. This approach is essential for bridging preclinical research and clinical translation.
Why choose Ubigene?
Choosing Ubigene means opting for a more efficient and precise CRISPR in vivo screening solution.
Comprehensive End-to-End Services
Ubigene possesses a robust pre-experimental optimization and quality management system, enabling the provision of one-stop, end-to-end in vivo CRISPR library screening services. This ensures seamless integration across all workflow stages.
Optimized Cell Pool Preparation Process
The end-to-end optimized Cell Pool production process enables large-scale, standardized manufacturing of library Cell Pools, ensuring minimal batch-to-batch variation and high reproducibility. Currently, over 400 screening-ready Cell Pools are available, with coverage rates reaching up to 99%.
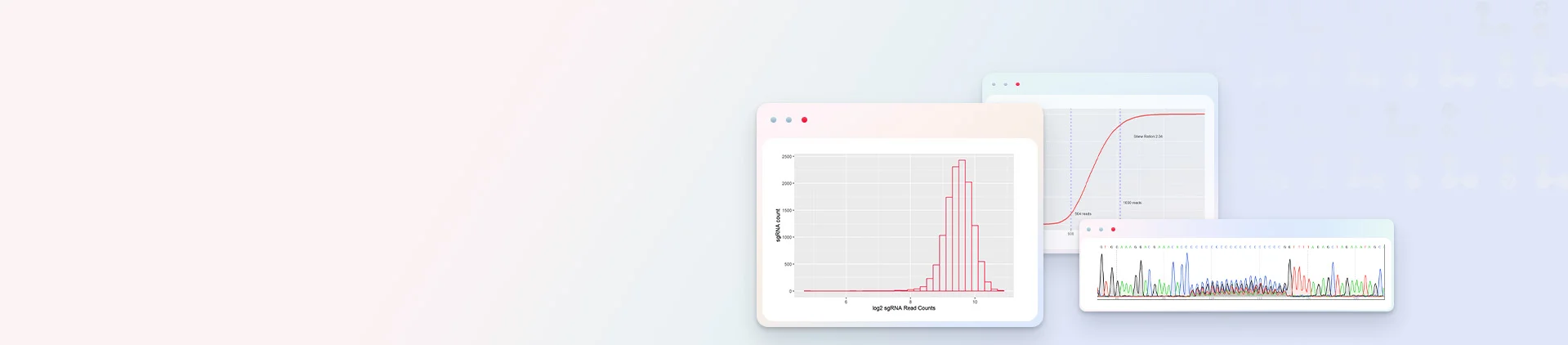
iScreenAnlys™ CRISPR Library Analysis Platform
Unlock intelligent library analysis for free with zero-code operation. Simply upload your sequencing data, and the platform automatically performs quality control, MAGeCK-based statistics, and pathway enrichment analysis. It directly generates ranked target heatmaps and differential gene volcano plots, providing publication-ready results for manuscript preparation.
Extensive Expertise
Leveraging extensive screening experience across tumor, immunology, and drug-resistance models, Ubigene can adapt flexibly to diverse research contexts, ensuring reliable and translatable results.
Workflow
CRISPR Library Cell Pool Construction

Preliminary Tumor Formation test

Tumor Cell Engraftment/ (Optional) compound Treatment

Tumor Tissue Collection

NGS Sequencing & Analysis
CRISPR Library Cell Pool Construction
Preliminary Tumor Formation test
Tumor Cell Engraftment/ (Optional) compound Treatment
Tumor Tissue Collection
NGS Sequencing & Analysis
CRISPR In Vivo Screening Service
In Vivo Tumor Formation with CRISPR Library Cell Pool
Subcutaneous implantation of CRISPR library cell pool, followed by tumor growth and harvesting of tumor tissues for NGS analysis.
Turnaround
8-12 weeks
Deliverables
NGS analysis report and associated mouse tumor formation experimental data.
CRISPR Library Cell Tumor compound Screening
Subcutaneous or orthotopic implantation of CRISPR library cell pool; after tumor formation, mice are randomized into treatment groups. Tumor tissues are harvested post-drug treatment for NGS analysis.
Turnaround
8-16 Weeks
Deliverables
NGS analysis report and associated mouse tumor formation experimental data.
CRISPR Library Cell Tumor Metastasis Study
Orthotopic or tail vein injection of CRISPR library cell pool; tumor cells colonize secondary organs. Tumor tissues from primary and metastatic sites are collected for NGS analysis.
Turnaround
12-20 Weeks
Deliverables
NGS analysis report and associated mouse tumor formation experimental data.
Successful CRISPR In Vivo Screening: Case Studies
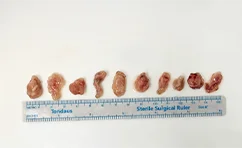
Tumor tissue collection in mice
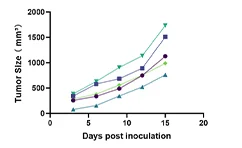
Tumor progression in mice
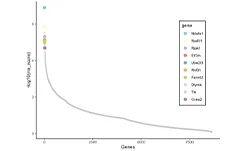
Bioinformatics analysis results Rank view of negative selection
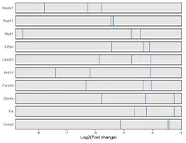
Bioinformatics analysis results
Representative publications
Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis
IF=42.5
Cell
Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis
In this study, the authors employed a CRISPR-Cas9–mediated genome-wide knockout screening system to investigate the genetic basis of tumor growth and metastasis. Using a genome-wide library containing 67,405 sgRNAs, they performed large-scale in vivo functional screens on a non-metastatic mouse cancer cell line. Following transplantation of the constructed cell pool into immunodeficient mice, metastatic lesions were rapidly induced. By evaluating sgRNA enrichment in lung metastases and late-stage primary tumors, and performing correlation analyses, the study revealed that loss-of-function mutations in specific genes drive tumor growth and metastatic progression. Validation of individual significant sgRNAs, or screening using a sublibrary of 624 sgRNAs targeting top-ranked genes from the primary screen, both markedly accelerated metastasis. This study further demonstrates that in vivo CRISPR screening is a highly effective approach for systematically dissecting gene-phenotype relationships during cancer progression. View details
View Picture
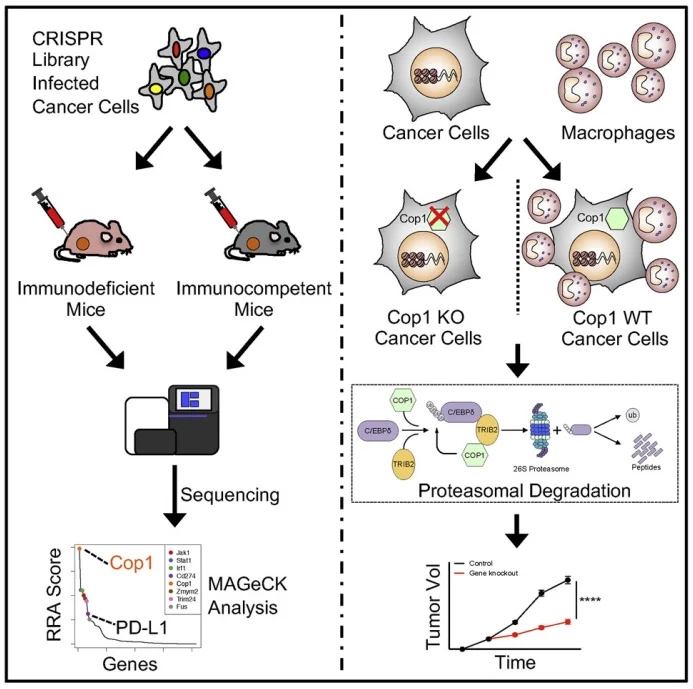
In vivo CRISPR screens identify the E3 ligase Cop1 as a modulator of macrophage infiltration and cancer immunotherapy target
IF=42.5
Cell
In vivo CRISPR screens identify the E3 ligase Cop1 as a modulator of macrophage infiltration and cancer immunotherapy target
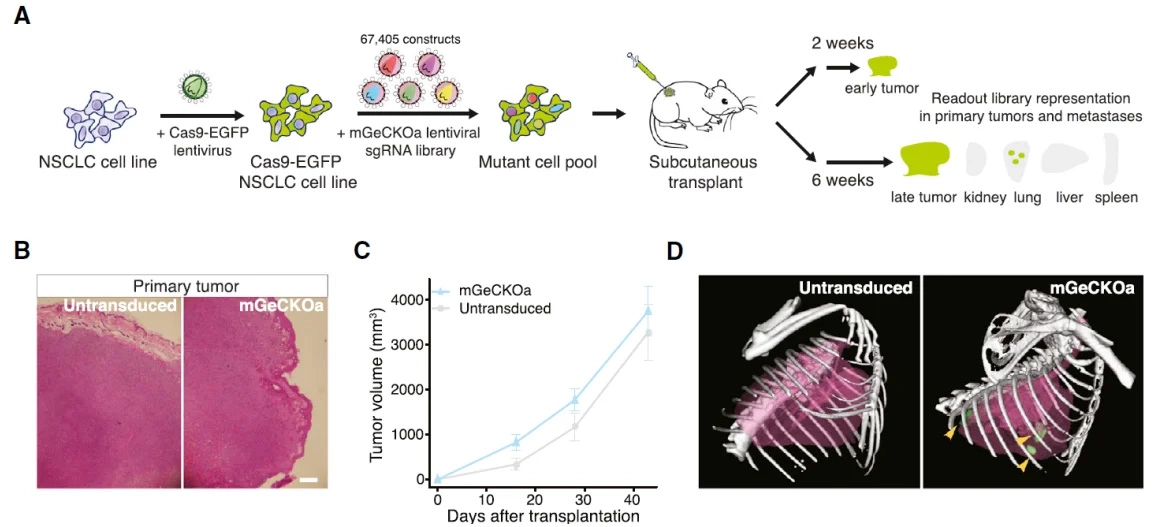
Although immune checkpoint blockade (ICB) has demonstrated remarkable clinical efficacy in cancer therapy, its therapeutic effect in triple-negative breast cancer (TNBC) remains limited. In this study, the authors performed in vivo CRISPR screening in mice to identify key proteins that modulate TNBC response to immunotherapy, revealing the E3 ubiquitin ligase Cop1 as a critical regulator. They further elucidated both intracellular and extracellular mechanisms by which Cop1 controls breast cancer progression. The authors first constructed a CRISPR/Cas9 screening library targeting approximately 4,500 mouse genes and used it to infect the 4T1 mouse TNBC cell line. Following orthotopic injection, TNBC tumor tissues were collected 16 days post-inoculation, and individual tumors were subjected to NGS sequencing. Bioinformatics analysis identified a series of candidate genes potentially regulating TNBC response to immunotherapy.To enhance the reliability of the in vivo CRISPR/Cas9 screen, approximately 80 candidate genes were selected to construct a second-round screening library. Through this iterative screening, the study ultimately identified the E3 ubiquitin ligase Cop1 as a key target regulating TNBC therapy.
View Picture


Contact Us
Simply fill out the form below to leave your inquiry
— we will respond within 24 Hours
* Name
* Institution
* Products & Services of Interest
If email is not available, how else can we reach you?
How did you hear about us?



