CRISPR Library Targets Lipid Metabolism Genes: New Targets for Cancer Metastasis


CRISPR Library Targets Lipid Metabolism Genes: New Targets for Cancer Metastasis

Research Background
Metastasized tumour cells poses a lethal threat to cancer patients. In order to escape and extravasate, necessary mutations in the tumour cell’s lipidome must occur to allow for promoted capability of transferring and anchoring. However, it remained unclear how tumourous cells’ lipidome contribute to such enhancement in metastatic potential.
Tonight, Ubigene brings forth a study on how “ACSL4 and polyunsaturated lipids support metastatic extravasation and colonization”. This newly published paper on Cell applies Lipidomic Analyses and CRISPR Library Screening in synergy, to demonstrate how Poly Unsaturated Fatty Acids (PUFA) can affect metastasis.
CRISPR Library Screening
Library: Human Metabolic Gene Knockout Library Screening
Method: In vivo screening
Screening Strategy:
1.Conduct in vivo screening using two different cancer cell lines with high metastatic potential.
2.After the first round of screening with the human metabolic gene knockout library, select potential target genes for in-depth secondary screening.
3.Collect tumor tissues post-cancer cell metastasis to identify metastasis-related targets.
Results
1.Combined Analysis of Cancer Cell Metastatic Potential and Ferroptosis Susceptibility
Using the MetMap (Cancer Metastasis Map) and CTRP (Cancer Therapeutics Response Portal) datasets for combined analysis, the study found that cancer types with high metastatic potential (such as ovarian cancer, liver cancer, and head and neck cancer) are more susceptible to ferroptosis.
Lipidomics studies based on liquid chromatography–tandem mass spectrometry (LC-MS/MS) have revealed that, compared with primary tumor cells from patients, metastatic tumor cells exhibit higher levels of polyunsaturated fatty acid (PUFA) lipids. One of the key hallmarks of ferroptosis is lipid peroxidation, with PUFAs being the main substrates for this process. These findings suggest that ovarian cancer cells with strong metastatic potential may be more susceptible to ferroptosis due to their higher levels of PUFAs.
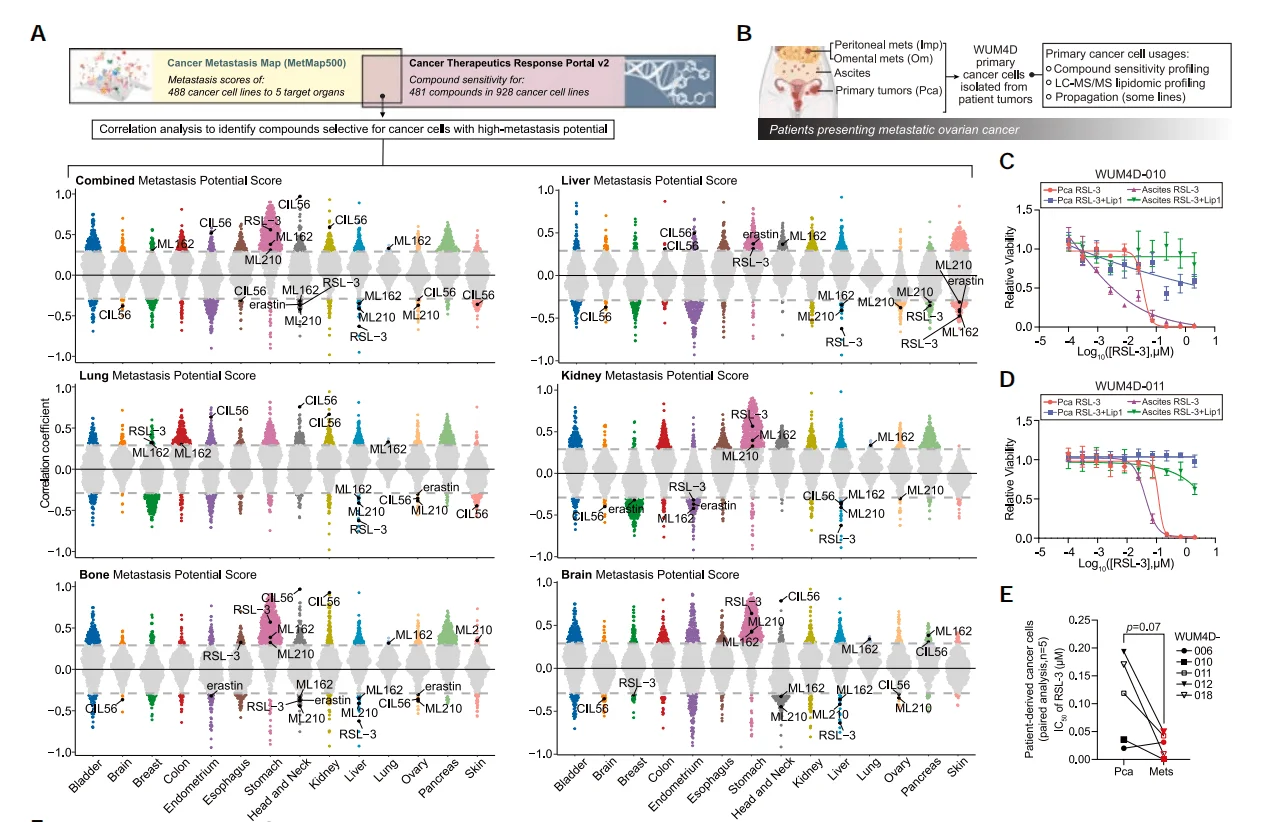
Figure 1. Combined analysis reveals an association between ferroptosis and cancer cell metastatic potential
2.CRISPR Library Screening Identifies Genes Promoting Cancer Cell Metastasis
Metabolic adaptation is crucial for cancer metastasis. To identify genes associated with cancer metastasis, researchers constructed an ovarian cancer ES-2 library cell line using a human metabolic gene knockout library and performed in vivo screening. After subcutaneous injection of the ES-2 library cells, they compared the differences between tumor cells that metastasized to the liver and lung and the original subcutaneous tumor cells. They found that several genes related to membrane lipid metabolism and fatty acid oxidation, such as TM7SF2, PHYH, PIK3CG, and PLA2G4C, were significantly enriched in both liver metastatic tumors and lung metastatic tumors.
To further validate the initial screening results, researchers conducted a second round of screening using the top 100 genes that were significantly enriched or depleted in the first screening. Comparative analysis revealed that the genes NMNAT1 and HADHB were significantly depleted in both liver and lung metastatic tumors. Additionally, ACSL4, a gene involved in unsaturated fatty acid metabolism, was also significantly depleted in lung metastatic tumors. Correlation analysis further showed that ovarian cancer cells with higher expression levels of the ACSL4 gene exhibited greater metastatic potential.
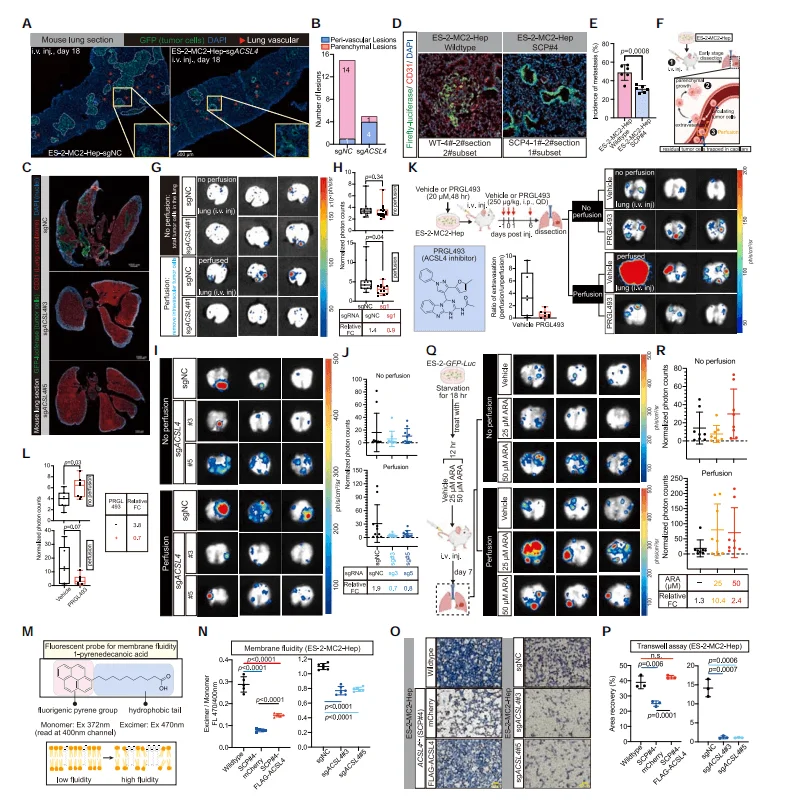
Figure 2. CRISPR Library Screening Identifies Genes Regulating Cancer Cell Metastasis
3.ACSL4 Promotes Cancer Cell Extravasation During Hematogenous Metastasis
To confirm the role of the ACSL4 gene in cancer cell metastasis, researchers injected ACSL4 knockout ES-2 cells via the tail vein and, in combination with immunofluorescence, found that the metastatic lesions formed by the intravenously injected ACSL4 knockout ES-2 cells were fewer and smaller. Moreover, these lesions exhibited an unusually high frequency of intravascular localization, indicating impaired extravasation function. As a key enzyme in the synthesis of unsaturated fatty acids, researchers speculated that ACSL4 knockout might impair cell membrane fluidity. Using fluorescent probes and Transwell assays, the study found that ACSL4 knockout significantly reduced cell membrane fluidity and affected cell invasiveness. However, pre-treatment with ARA to increase the PUFA content of cells could enhance the metastatic ability of intravenously injected ACSL4 knockout ES-2 cells. These results suggest that ACSL4 promotes metastatic extravasation by regulating the PUFA content of the cell membrane, thereby enhancing the membrane fluidity and invasiveness of disseminated cancer cells.
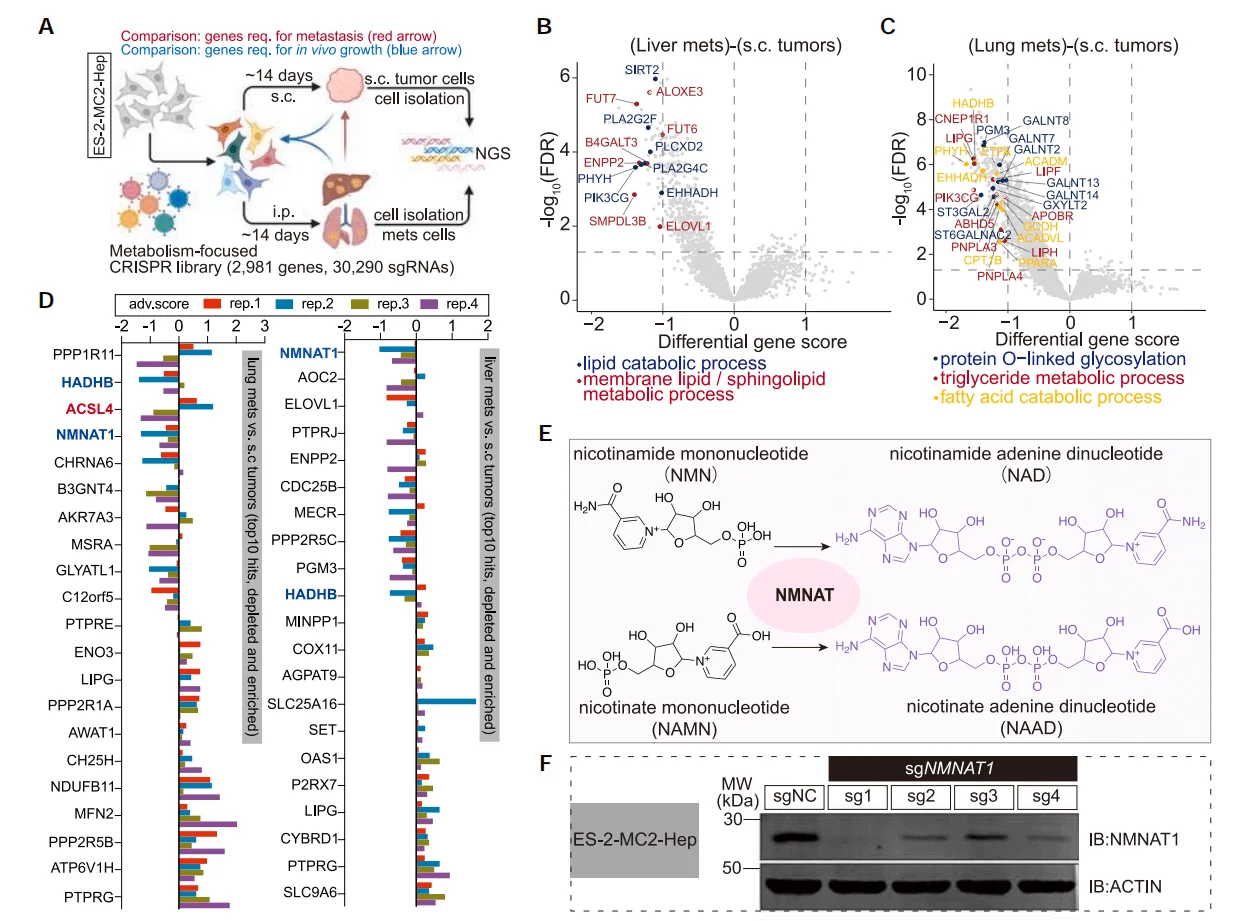
Figure 3. ACSL4 Promotes Cancer Cell Metastatic Extravasation
4.ACSL4 Promotes Metastasis in Other Cancer Cells
Researchers further conducted CRISPR library screening using the highly metastatic hepatocellular carcinoma cell line SK-HEP-1 and similarly found that the genes NMNAT1 and ACSL4 were significantly depleted. Moreover, through ACSL4 gene knockout and ARA treatment to increase cellular PUFA content, they confirmed the promoting effect of unsaturated fatty acids on cancer cell metastasis.
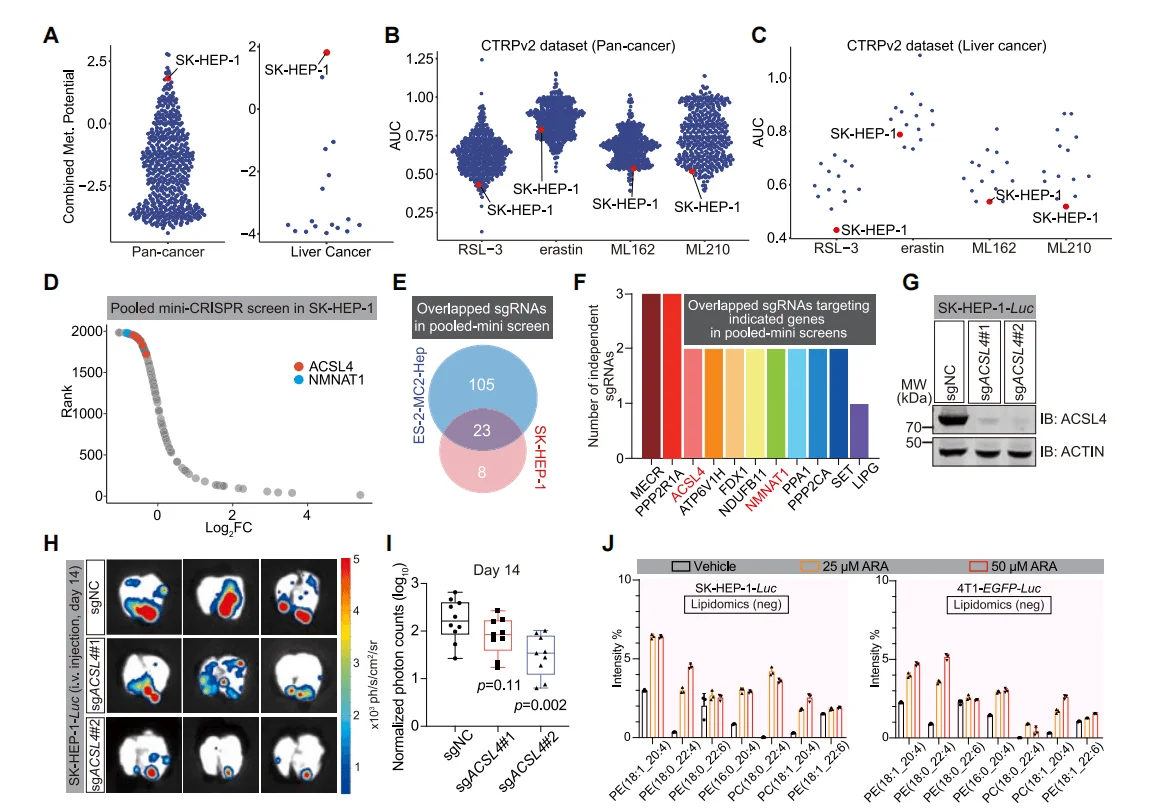
Figure 4. ACSL4 is Significantly Enriched in CRISPR Library Screening of Hepatocellular Carcinoma Cells
5.Dual Inhibition of ACSL4 and ECH1 Suppresses Cancer Metastasis
Although ACSL4 knockout can inhibit the metastasis of cancer cells, due to the highly aggressive nature of tumors, the metastatic burden in the later stages of the disease remains difficult to control. To explore methods for inhibiting tumor growth and metastasis, researchers compared the abundance of sgRNA in tumors post-implantation with that in cells prior to injection. They found that genes such as ECI1 and ECH1 were significantly depleted in various metastatic tumors, and that knockout of these genes also significantly inhibited the growth of cancer cells. Further, by constructing a dual-gene knockout cell line for ACSL4 and ECH1, the study found that compared with single-gene knockout cells, the pulmonary metastatic burden in the dual-gene knockout cells was significantly reduced.
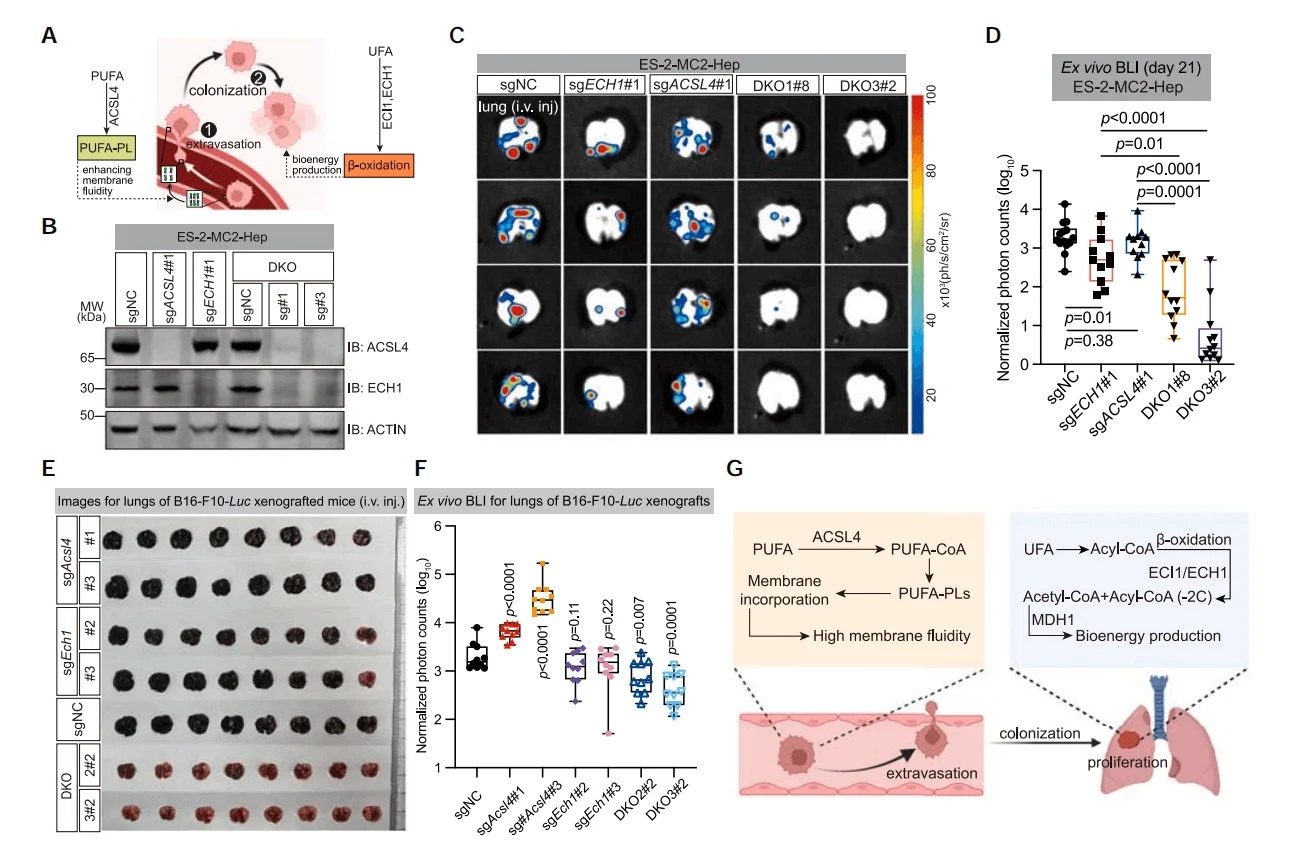
Figure 5. Dual Knockout of ACSL4 and ECH1 Suppresses Cancer Cell Metastasis
Conclusion
Through CRISPR screening, researchers identified ACSL4 as a key factor promoting hematogenous metastasis, which facilitates metastasis by enhancing cell membrane fluidity and invasiveness. Moreover, tumor cells with high PUFA content rely on ECI1/ECH1 for β-oxidation,and combined inhibition of ACSL4 and ECH1 can effectively suppress cancer metastasis.
Reference
Wang Y, Hu M, Cao J, Wang F, Han JR, Wu TW, Li L, Yu J, Fan Y, Xie G, Lian H, Cao Y, Naowarojna N, Wang X, Zou Y. ACSL4 and polyunsaturated lipids support metastatic extravasation and colonization. Cell. 2025 Jan 23;188(2):412-429.e27.


