Leveraging CRISPR Screening in Aging Research Models——Unlocking the “Reboot Code” of the Aging Brain: Toward Neur


Leveraging CRISPR Screening in Aging Research Models——Unlocking the “Reboot Code” of the Aging Brain: Toward Neural Stem Cell Regeneration
Research Background
The functional decline of human organs is an inevitable hallmark of aging, and the brain is no exception. As individuals age, sensory and cognitive functions gradually deteriorate, accompanied by an increased risk of neurodegenerative disorders, most notably Alzheimer’s disease and Parkinson’s disease.
Within the brain, specific regions such as the subventricular zone (SVZ) are enriched with neural stem cells (NSCs). In response to injury, these NSCs can transition from a quiescent state (qNSCs) to an activated state (aNSCs) with proliferative potential. Subsequently, they undergo tightly regulated processes of differentiation and migration to supply new neurons to damaged brain areas.
However, during aging, the activation capacity of NSCs declines significantly, impairing neurogenesis and leading to progressive deficits in sensory and cognitive functions. Identifying and characterizing the molecular regulators of NSC activation is therefore crucial for developing strategies to intervene in age-related neurodegenerative processes.
Although previous studies have identified several key factors that promote the activation of aged NSCs, a comprehensive and systematic investigation of these regulatory mechanisms remains lacking. Furthermore, CRISPR–Cas9 library-based high-throughput genetic screening, a powerful tool for functional genomics, has not yet been fully adapted or optimized for use in aged cells or in vivo aging models.
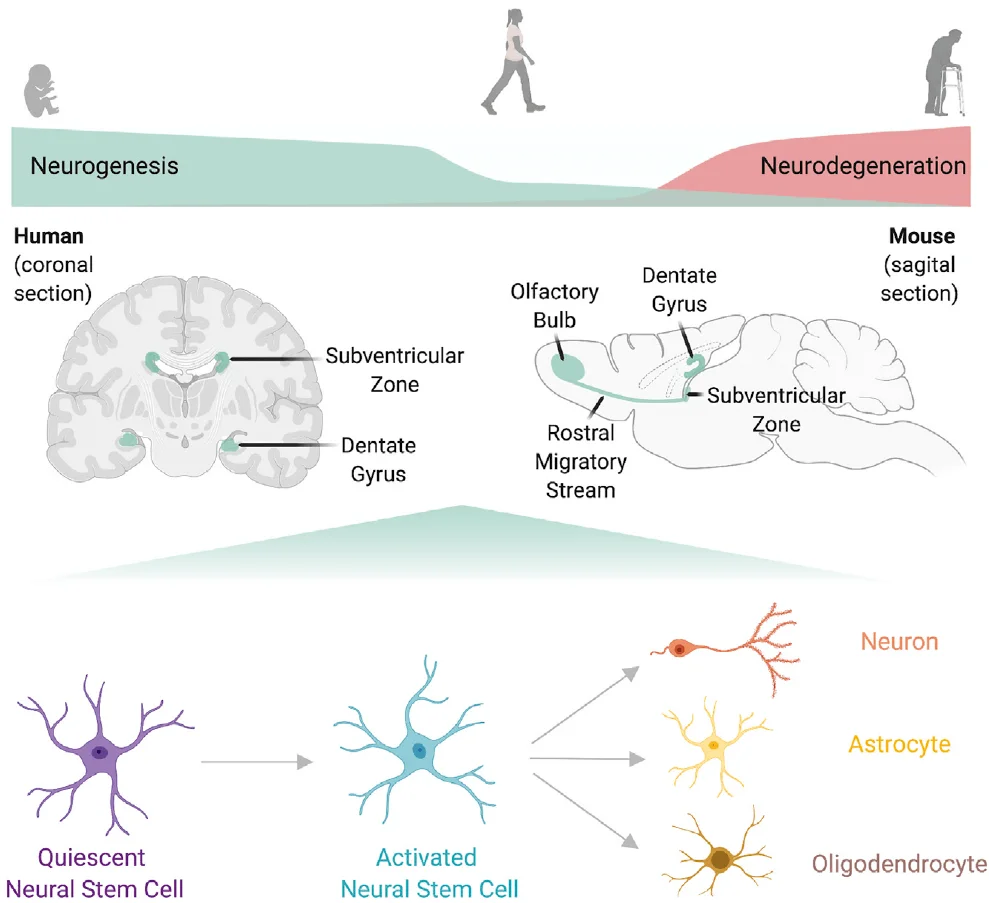
Figure 1. Brain regions and the activation and differentiation of neural stem cells [1]
Research Overview
On October 2, 2024, the research team led by Anne Brunet at Stanford University published a landmark study in Nature, entitled “CRISPR–Cas9 screens reveal regulators of ageing in neural stem cells” [2]. In this study, the authors developed both in vitro and in vivo high-throughput CRISPR–Cas9 screening platforms specifically designed for aging cells and organisms. By applying these platforms to aged neural stem cells (NSCs) in mice, they systematically identified genetic regulators that promote the activation of aged NSCs, and further performed comprehensive functional validation of these candidate genes.

FigureCRISPR–Cas9 screens reveal regulators of ageing in neural stem cells
To comprehensively identify genes that regulate the activation of aged neural stem cells (NSCs), the authors isolated primary NSCs from both young and aged mice and performed a CRISPR–Cas9 library screen on these cells. Under defined growth factor conditions, cultured NSCs can transition from a quiescent state (qNSCs) to an activated state (aNSCs). Leveraging this property, the researchers expanded NSCs in vitro and introduced a genome-wide knockout library targeting approximately 23,000 protein-coding genes via lentiviral transduction, achieving successful infection of more than 400 million qNSCs.
The qNSCs were subsequently activated with growth factors, and cell populations were collected at two critical time points: day 4, when successfully activated NSCs were enriched by fluorescence-activated cell sorting (FACS), and day 14, when cells were enriched based on the proliferative advantage of activated NSCs. By analyzing sgRNA enrichment and depletion patterns through deep sequencing, the authors identified 301 gene knockouts that specifically enhanced the activation of aged NSCs. Functional annotation revealed that these genes were highly enriched in pathways related to ciliary structure, RNA-binding proteins, and glucose transport.
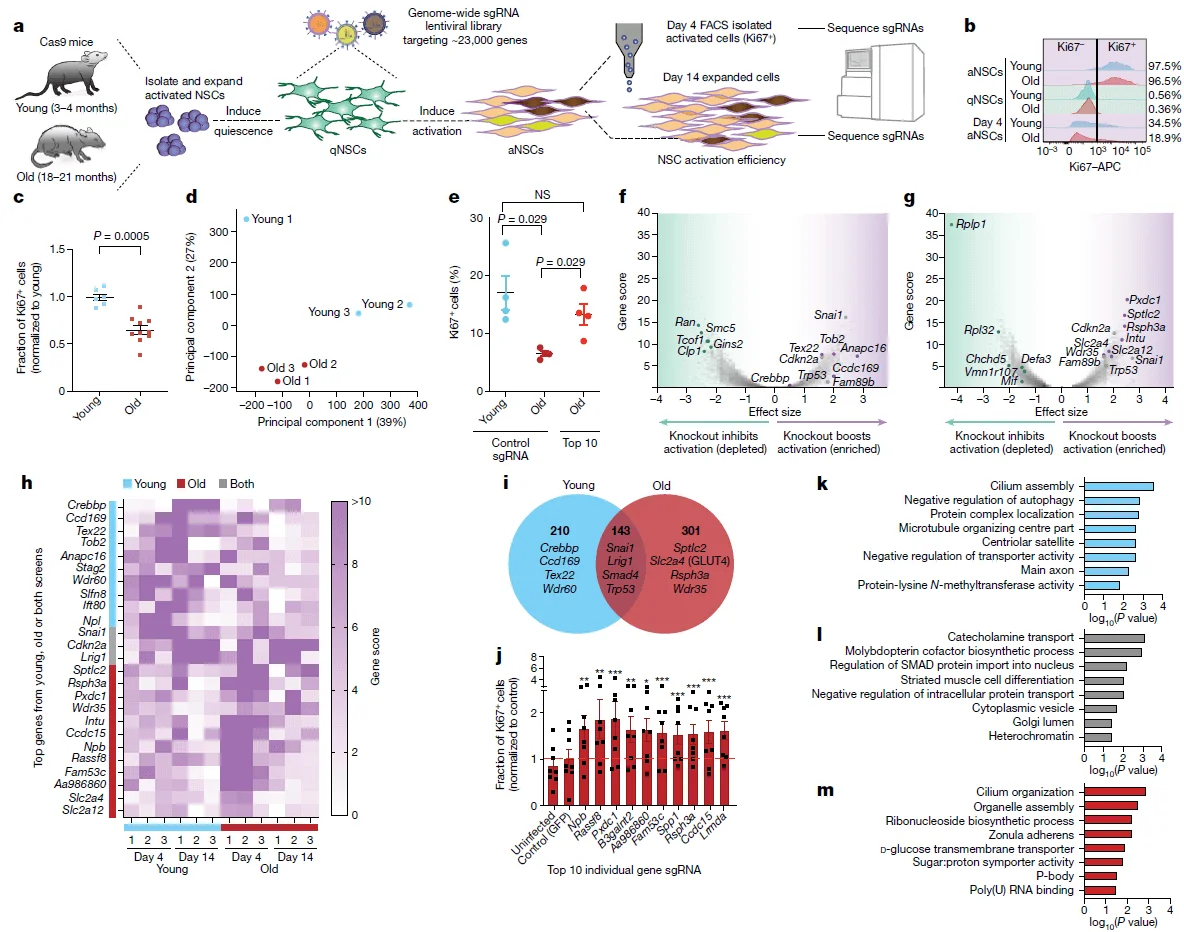
Figure 2. In vitro CRISPR–Cas9 screening for regulators of aged neural stem cell (NSC) function
Although the in vitro system can partially recapitulate aspects of the aging process, it does not fully reflect the complexity of the in vivo environment. To validate the functional targets identified from the in vitro CRISPR screens—genes whose knockout enhanced NSC activation—the authors further developed an in vivo CRISPR–Cas9 screening platform in the brains of aged mice.
In the subventricular zone (SVZ), quiescent NSCs (qNSCs) can be activated and generate progeny that migrate to the olfactory bulb, where they differentiate into newborn neurons. This intrinsic regenerative pathway provides an ideal biological model for in vivo functional screening.
The authors injected sgRNA library viruses into the lateral ventricles of aged mice, targeting NSCs in the SVZ, and harvested genomic DNA from the olfactory bulb 5 weeks post-infection for sequencing analysis. Based on the results from the in vitro screens, they evaluated five customized sgRNA libraries (10 genes per library), including:
- 1. The “Top 10” hits whose knockout enhanced aged NSC activation in vitro;
- 2. Genes involved in glucose uptake and human disease pathways;
- 3. Genes related to cytoplasmic ribonucleoprotein structure;
- 4. Representative genes whose knockout inhibited NSC activation in vitro;
- 5. Known positive regulators of NSC activation.
Through this in vivo screening, 24 genes were significantly enriched in the olfactory bulb tissue. Notably, 9 out of 10 genes in the glucose uptake and human disease–related library showed significant enrichment in at least one in vivo screen, highlighting their robust functional impact on aged NSC activation.
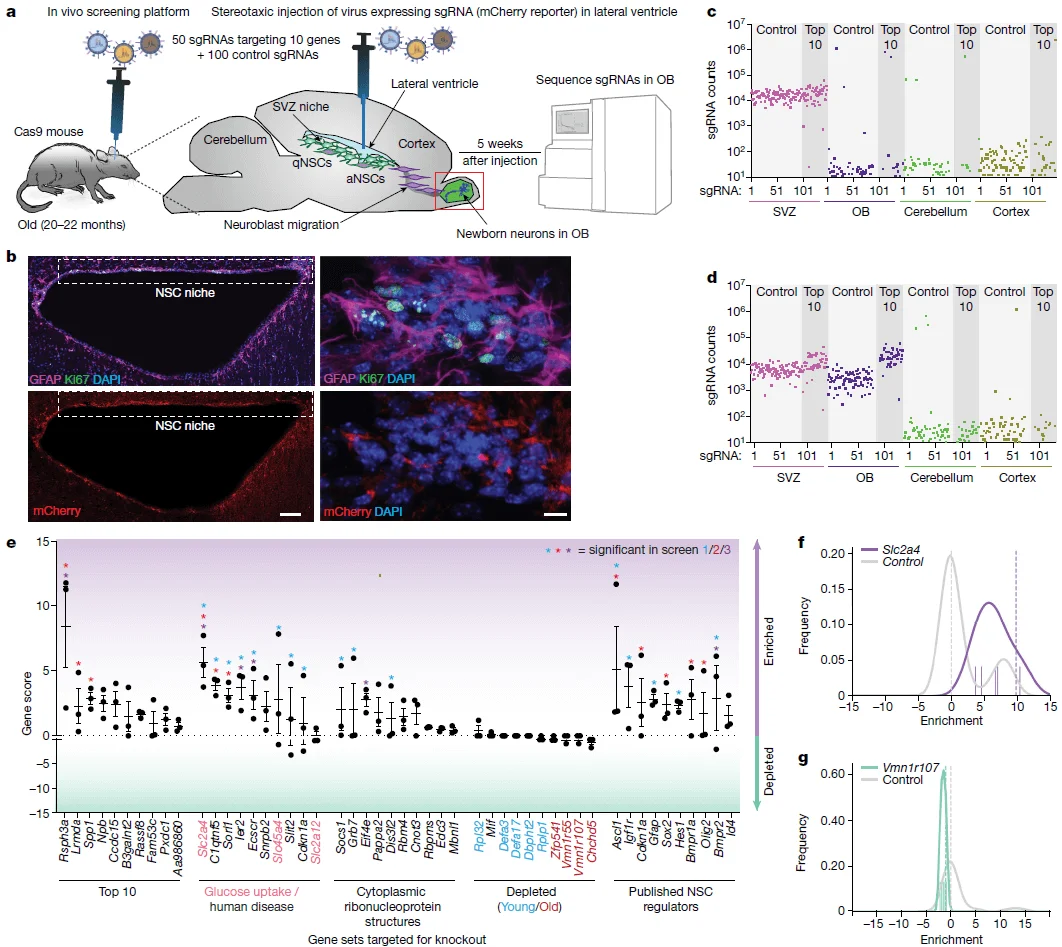
Figure 3. In vivo CRISPR–Cas9 screening in the aged mouse brain
In both in vitro and in vivo CRISPR screens, knockout of the Slc2a4 gene—which encodes the glucose transporter GLUT4—consistently enhanced the activity and functional capacity of aged NSCs. While previous studies have implicated glucose metabolism in regulating self-renewal, survival, and differentiation of young NSCs, the role of GLUT4 in aged NSCs and in the aging brain remained largely unexplored.
To address this, the authors performed downstream functional validation of Slc2a4. They found that Slc2a4 knockout led to a significant increase in the number of newborn neurons in the olfactory bulb 5 weeks after viral infection. Furthermore, knockout of Slc2a4 also resulted in increased numbers of both quiescent and activated NSCs in the subventricular zone (SVZ). Consistent with these findings, GLUT4 expression in NSCs was observed to increase with age, suggesting a potential age-dependent inhibitory effect of GLUT4 on NSC activation.
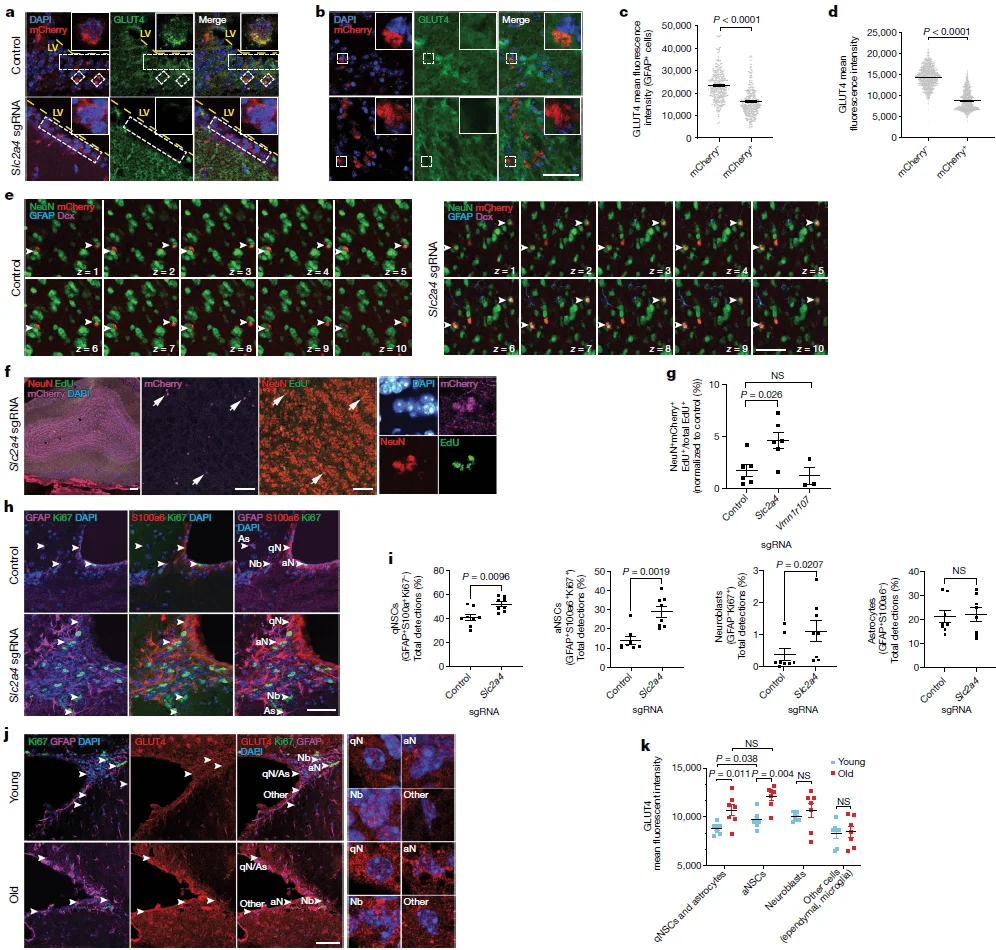
Figure 4. Functional validation of Slc2a4 gene knockout
Based on these functional validations, the authors proposed that the age-dependent upregulation of GLUT4 may strongly inhibit the activity and functional capacity of NSCs in the aging brain. GLUT4 is an insulin-dependent glucose transporter that facilitates cellular glucose uptake.
Beyond genetic manipulation, the authors also tested whether limiting glucose availability could modulate the activity and function of aged NSCs. Their results demonstrated that both transient glucose deprivation and interference using glucose analogs effectively activated aged NSCs. These findings suggest that reducing glucose uptake in NSCs may represent a promising strategy to restore the activation potential and regenerative capacity of aged NSCs.
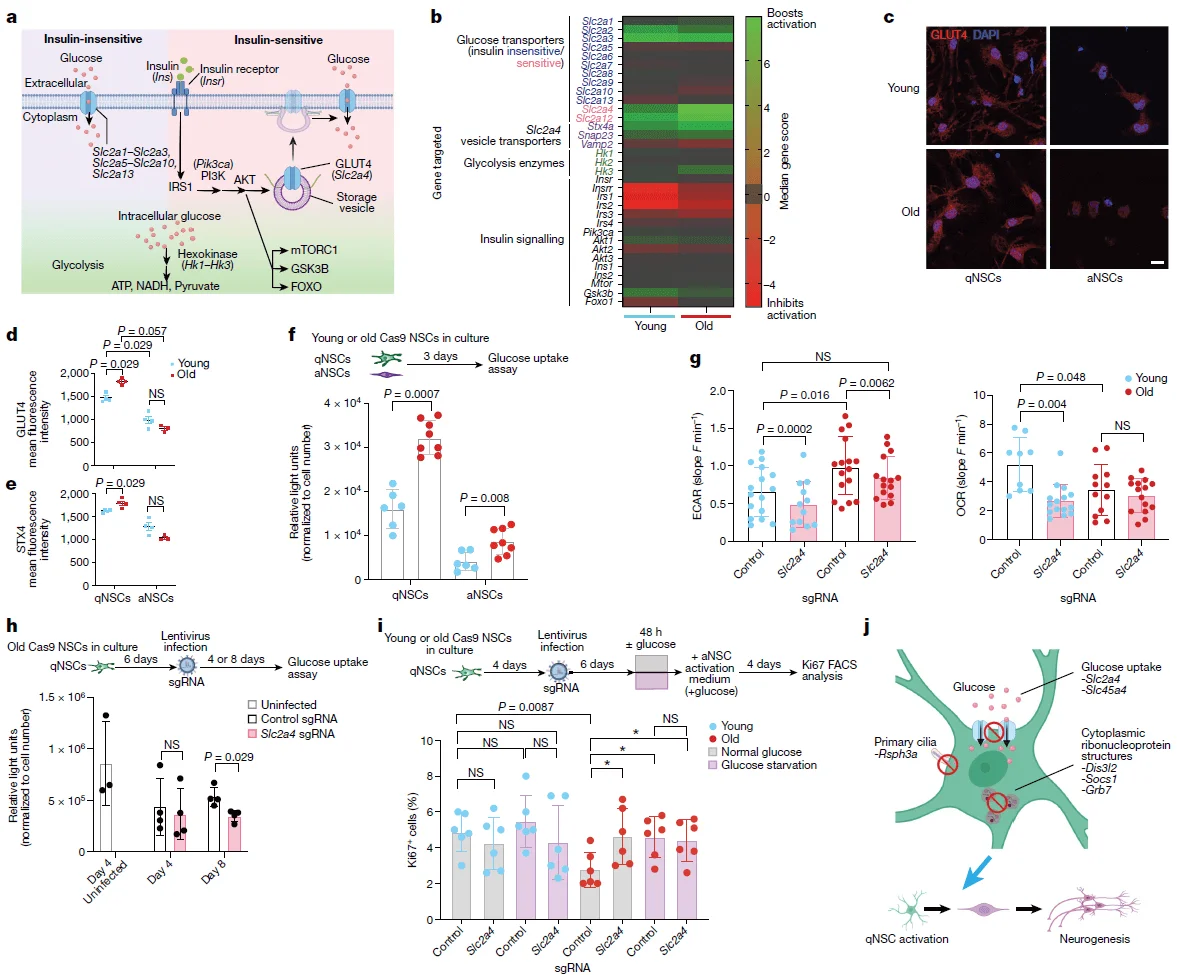
Figure 5. Effects of glucose uptake on the function of aged neural stem cells (NSCs)
Conclusions and Significance
In this study, the authors developed high-throughput in vitro and in vivo CRISPR–Cas9 screening platforms to systematically identify regulators that promote aged neural stem cell (NSC) activation and neurogenesis. Through genome-wide in vitro screening of primary NSCs from both young and aged mice, they identified 301 gene knockouts that specifically enhanced the activation of aged NSCs. These genes were enriched in biological processes such as ciliary organization and glucose uptake.
In addition, the authors established an expandable in vivo CRISPR–Cas9 screening platform, which identified 24 gene knockouts that promoted NSC activation and function in the aged mouse brain. Notably, knockout of Slc2a4 (encoding the GLUT4 glucose transporter) was demonstrated to be an effective intervention for improving aged NSC function. With aging, NSCs exhibit increased glucose uptake, and limiting glucose availability was shown to enhance the activation potential of aged NSCs.
These findings not only deepen our understanding of NSC aging mechanisms, but also provide a potential foundation for developing novel therapeutic strategies against age-related neurodegenerative diseases.
Key Highlights of the CRISPR Screening Strategy
- 1. Combined validation of in vitro high-throughput screening with expandable in vivo platforms;
- 2. Ultra-large-scale, high-coverage genome-wide knockout screens;
- 3. Dual time-point analysis at day 4 (FACS enrichment for activated NSCs) and day 14 (proliferation-based enrichment);
- 4. Parallel screening of young and aged NSCs to identify age-specific regulators of NSC activation;
- 5. Integration of in vitro NSC activation with the intrinsic regenerative properties of the in vivo system to provide an effective functional screening framework
Research and Application Support: Ubigene Stem Cell Tools Portfolio
To support researchers in advancing stem cell gene editing and aging studies, Ubigene offers the following high-quality products and services:
- · EZ-Stem™ Cell Culture Medium(#YMH-003)
- · Human induced pluripotent stem cells (iPSC)(IPSC-DYR0100, #YC-C098)
- · iPSC Gene Editing Services
- · iPSC Differentiation Services
Conclusion
The introduction of CRISPR library screening provides a novel perspective and powerful tools for aging research and neural stem cell regeneration. Through large-scale functional genomic screens, researchers can not only systematically identify key regulatory factors involved in NSC aging, but also validate intervention effects at the organismal level.
As these platforms and technologies continue to mature, targeting critical pathways such as metabolism, ciliary function, and RNA regulation holds promise for “rebooting” the aging brain. Such approaches may ultimately slow cognitive decline and offer new therapeutic avenues for neurodegenerative diseases.
Reference
- [1] Navarro Negredo P, Yeo RW, Brunet A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell. 2020 Aug 6;27(2):202-223.
- [2] Ruetz TJ, Pogson AN, Kashiwagi CM, Gagnon SD, Morton B, Sun ED, Na J, Yeo RW, Leeman DS, Morgens DW, Tsui CK, Li A, Bassik MC, Brunet A. CRISPR-Cas9 screens reveal regulators of ageing in neural stem cells. Nature. 2024 Oct;634(8036):1150-1159.



