How to Generate a Knockout (KO) Cell Line with CRISPR/Cas9


The CRISPR/Cas9 genome editing system has revolutionized molecular and cellular biology by enabling precise, efficient, and programmable loss-of-function mutations in virtually any gene of interest. By introducing targeted double-strand breaks (DSBs) and harnessing the cell's repair machinery, CRISPR facilitates the generation of knockout (KO) cell lines, providing invaluable tools for functional genomics, protein characterization, and target validation.
A single cell harboring the desired mutation can be expanded into a clonal KO cell line, which serves as a uniform and reproducible experimental system. These clonal lines are essential for understanding the functional consequences of gene loss, studying signaling pathways, and validating the specificity of biological reagents. However, despite the transformative potential of CRISPR, the derivation of high-quality KO clones is technically challenging. Success depends on careful experimental design, optimized delivery, clonal selection, and rigorous validation. Here, we provide a comprehensive overview of the standard workflow for generating CRISPR KO cell lines, highlighting key considerations and practical strategies.
Design and Production of KO Guides
The first critical step is the design of guide RNAs (gRNAs), which direct the Cas9 nuclease to the target genomic locus. Effective gRNA design determines the efficiency, specificity, and success of gene disruption.
Key steps include:
- Target selection: Identify exons critical for protein function to maximize the likelihood of complete knockout.
- gRNA design: Tools such as Red Cotton™ CRISPR Gene Editing Designer provide optimized gRNA candidates using cell-type-specific parameters for over 1600 cell lines. Multiple gRNAs targeting the same gene can be employed to increase editing efficiency and reduce false negatives.
-
Guide formats:
Options include single-guide RNA (sgRNA), dual crRNA:tracrRNA
systems, plasmid- or lentivirus-expressed guides, and in
vitro-transcribed RNA.
- Plasmid and lentiviral delivery are widely used but may be time-consuming and require selection agents; lentiviral delivery is advantageous for hard-to-transfect cells.
- sgRNAs are faster and often have reduced off-target effects.
Once designed, gRNAs are synthesized, cloned into expression vectors, and sequence-verified. For convenience, Ubigene maintains a Red Cotton™ gRNA plasmid bank containing over 10,000 validated knockout plasmids, ready for immediate use. Each plasmid is cost-effective (About $80) and fully compatible with standard CRISPR workflows.
Cell Transfection and Delivery of CRISPR Components
The next critical step is the efficient delivery of CRISPR components into target cells. Transfection efficiency is a major determinant of successful KO generation, and each cell type has unique challenges:
- Stem cells, primary cells, and hematopoietic or immune lineages often have lower transfection efficiencies and increased sensitivity to cytotoxic stress.
- Optimization of delivery method is often required, including electroporation, lipid-mediated transfection, or viral delivery, tailored to the cell type, growth conditions, and experimental goals.
Ubigene has extensive experience with over 300 cell types and maintains standardized protocols to determine the optimal delivery strategy, ensuring high editing efficiency while maintaining cell viability. With Ubigene's workflow, homozygous KO clones can be obtained in as fast as one week.
Enrichment of Edited Cells and Single-Cell Clonal Expansion
After successful editing, enrichment of edited cells and isolation of single-cell clones are crucial to establish a homogeneous KO cell line.
Two common approaches include:
- Limiting dilution cloning: Simple and inexpensive, but may take longer and sometimes yields fewer viable clones.
- Fluorescence-activated cell sorting (FACS): Allows precise isolation of double-positive or marker-expressing cells, increasing the likelihood of obtaining correctly edited clones.
During clonal expansion, growth conditions must be carefully optimized. Suboptimal conditions can result in loss of valuable clones or poor viability. For challenging cell lines, such as primary cells or lines with low proliferation rates, Ubigene performs preliminary tests to determine the best conditions for single-cell survival and expansion.
Confirmation and Validation of Gene Edits
Rigorous validation of gene edits is essential to ensure that the KO cell line is accurately engineered and suitable for downstream applications. Multiple complementary approaches are recommended:
-
Cellular Functional Validation:
- Sanger sequencing to verify specific indels at the target locus.
- Next-generation sequencing (NGS) for high-throughput confirmation and detection of potential off-target edits.
- PCR-based assays to assess gene disruption.
- Proteomic validation: Western blot and mass spectrometry to confirm the absence of protein expression.
- Functional validation: Immunocytochemistry (ICC), immunohistochemistry (IHC), or FACS-based assays to verify functional knockout at the cellular level.
By combining these approaches, researchers can confidently confirm a complete knockout, ensuring reproducibility and accuracy in downstream studies.
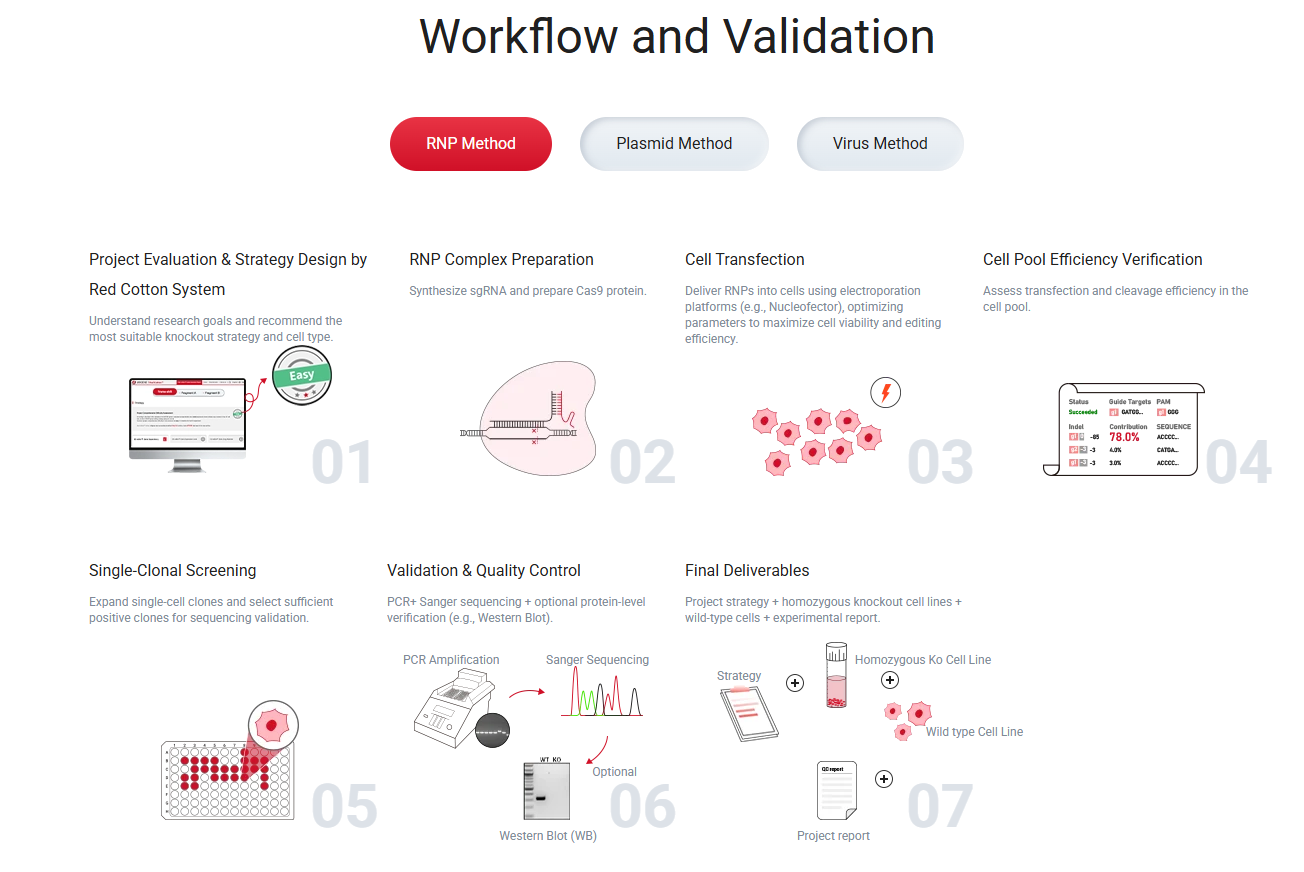
Figure 1. Overview of the CRISPR/Cas9 KO cell line generation workflow
Ubigene KO Cell Line Services and Products
Ubigene provides a comprehensive range of knockout (KO) cell line solutions to meet diverse research needs. Ready-to-use KO cell lines are available for as low as $990 with a turnaround time of just 1 week for a KO homozygous clone. For more specialized requirements, customized KO cell line generation is offered starting at $2,980, with a typical turnaround of 4-6 weeks, depending on the cell type and project complexity.
Conclusion
Generating a CRISPR-mediated knockout cell line requires careful planning, optimized transfection, precise clonal isolation, and rigorous validation. With expertise and advanced tools such as Red Cotton™ gRNA plasmid bank and Ubigene's optimized workflows, researchers can reliably create high-quality, reproducible KO clones. These cell lines are invaluable for functional genomics, drug target validation, and mechanistic studies, forming a cornerstone of modern biomedical research.
Whether you are seeking ready-made KO clones or customized KO cell line services, Ubigene provides fast, efficient, and high-quality solutions to accelerate your research from concept to discovery.
Contact us to learn more>>>

