Literature Interpretation: Breakthrough Discovery in Reovirus Infection Mechanism - The PirB Receptor


Literature Interpretation: Breakthrough Discovery in Reovirus Infection Mechanism - The PirB Receptor

Abstract
In 2023, a study at the University of Pittsburgh brought forth Paired Immunoglobulin-like Receptor B (PirB), a key receptor responsible for the entry of reovirus into host cells. With the help of high-passaging CRISPR-activation (CRISPR-a) screening, deep sequencing, flow cytometry, and atomic force microscopy (AFM), researchers were able to identified the D3D4 extracellular ectodomains as a essential for reovirus binding and infection.These findings were further validated through in vivo murine experiments. These findings expanded our understanding of reovirus, while providing new insights for strategies targeting viral entry. PirB has also been implicated in a series of viral-induced inflammation, which suggesting potential for new immunotherapies with a solid theoretical foundation.
Background
Reovirus is a pathogen with a wide spectrum of mammalian hosts, including humans. It can result in several CNS disorders, such as encephalitis and meningitis. Reovirus infects its host through a complex set of processes involving receptor binding and internalization. These interactions determine several characteristics of the corresponding disease, such as host range, means of transmission, cell and tissue tropism, and severity. Known interactions between the σ1 protein, sialic acid (SA), and junctional adhesion molecule A (JAM-A) cannot fully explain the serotype-specific CNS tropism and diseases. Thus, researchers need to identify new receptors to better understand the features of reoviruses.
Results
CRISPRa screening recognizes PirB as candidate
PirB was identified as a main candidate following a whole-genome CRISPR-a screening in JAM-A−/−/NgR1−/− double-knockout (DKO) mouse embryonic fibroblast (MEF) cell line. PirB, a member of the LILR family, is widely expressed in immune cells and neurons. It serves as a major binding site for major histocompatibility complex class I (MHC-I) proteins and myelin-associated inhibitors (MAIs).
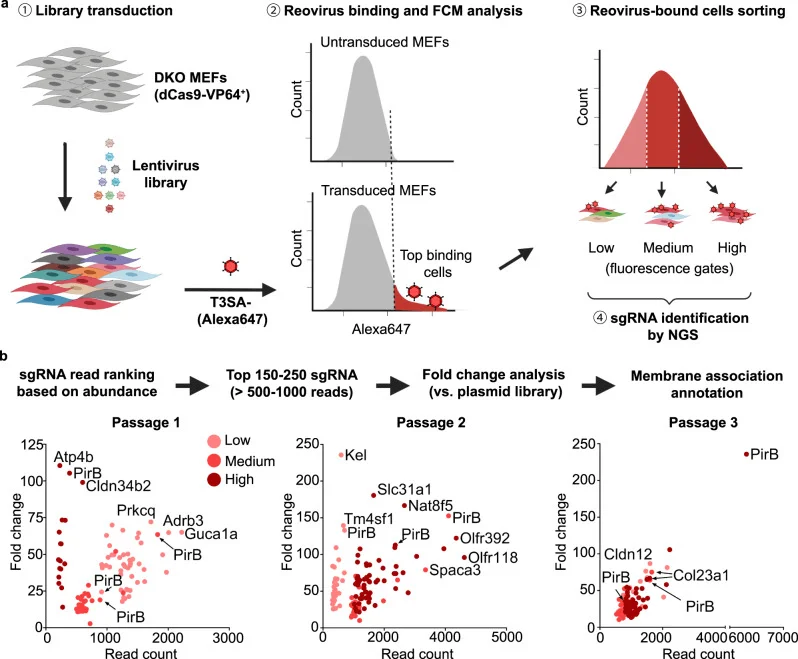
Figure 1 CRISPRa screen for reovirus receptors identifies PirB as a candidate
Validation of PirB Function: Inducing Reovirus Binding and Infection
To verify PirB’s role as a reovirus receptor, researchers expressed exogenous PirB in minimally susceptible Chinese Hamster Ovary (CHO) cells to examine the binding and infection capabilities of reovirus. The results showed a significant increase in reovirus binding affinity and infection efficiency upon the introduction of PirB.
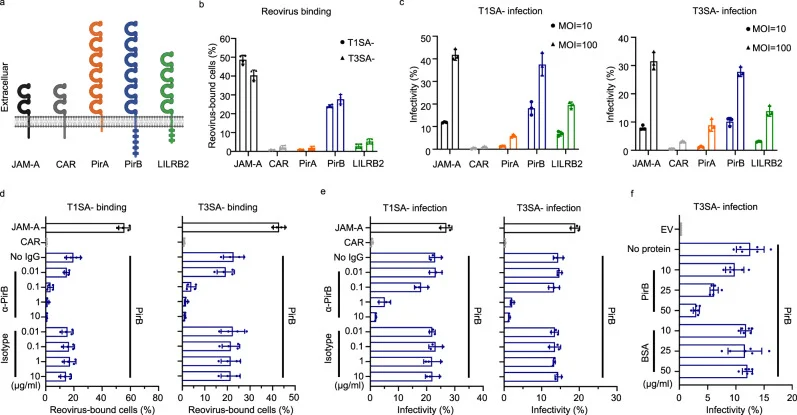
Figure 2 PirB promotes reoviral binding and infectivity
The D3D4 domain of PirB is a key factor for reovirus binding and infection.
A combination of chimeric receptor proteins, in which the homologous ectodomains of PirA and PirB were swapped, displayed varying infection profiles of the reovirus. Differential analysis demonstrated that reovirus infection was severely hindered only when the D3D4 ectodomain was swapped out of PirB, providing evidence that the D3D4 domain is a vital region for reovirus binding and infection.
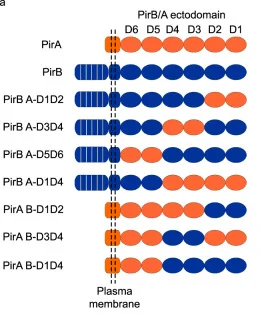
Figure 3 PirB D3D4 is required for reovirus binding and infection
AFM results showed a highly specific interaction between reovirus and PirB, with an equilibrium dissociation constant (KD) of 1.94 ± 1.72 nM. Besides, the reoviral outer-capsid protein σ3 was suggested to be the ligand for PirB binding.
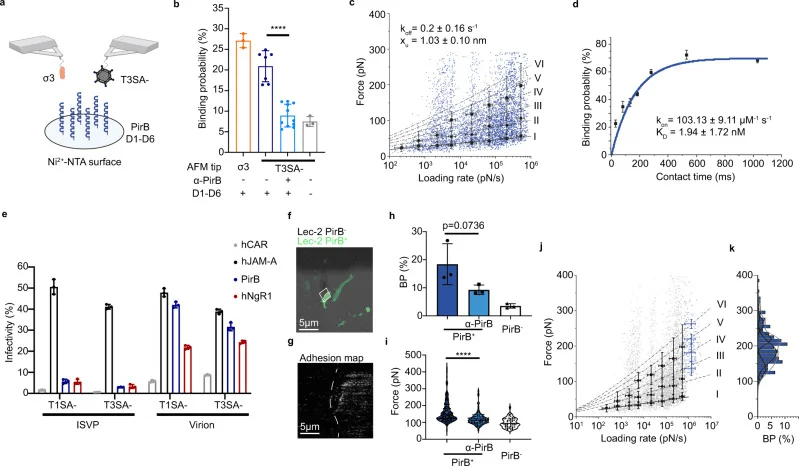
Figure 4 Biophysics of the reovirus and PirB interaction
PirB signaling cascades facilitate viral internalization.
PirB’s intracellular domain (ICD) consists of multiple immunoreceptor tyrosine-based switch motifs (ITSMs). Experiments showed that reovirus binding activates intracellular PirB signaling, which in turn aids in the internalization of the reovirus. Such vital induction of signaling may be mediated by SHP-1/2 phosphatases, thereby promoting the internalization of reoviruses.
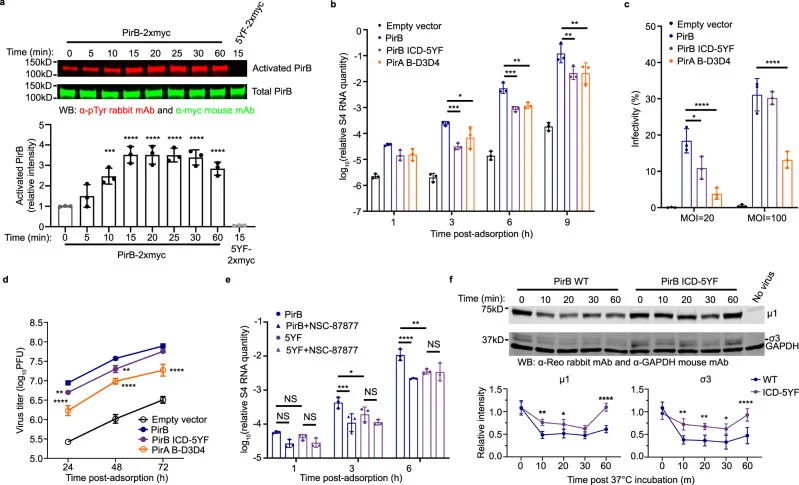
Figure 5 PirB intracellular signaling is required for efficient reovirus entry
PirB drives neuropathogenicity in murine models.
Replication and pathogenicity of T3 reovirus were found to be vastly lowered in PirB-/- mice compared to WT mice. The neural-specific Knockout model (NspPirB-/-) also showed, in concordance, the importance of PirB expression in neuropathogenicity by T3 reovirus. Furthermore, primary cortical neurons treated with PirB-specific antibody exhibited decreased reoviral infections, while T3 reovirus replicated at a significantly attenuated rate in PirB-/- neurons.
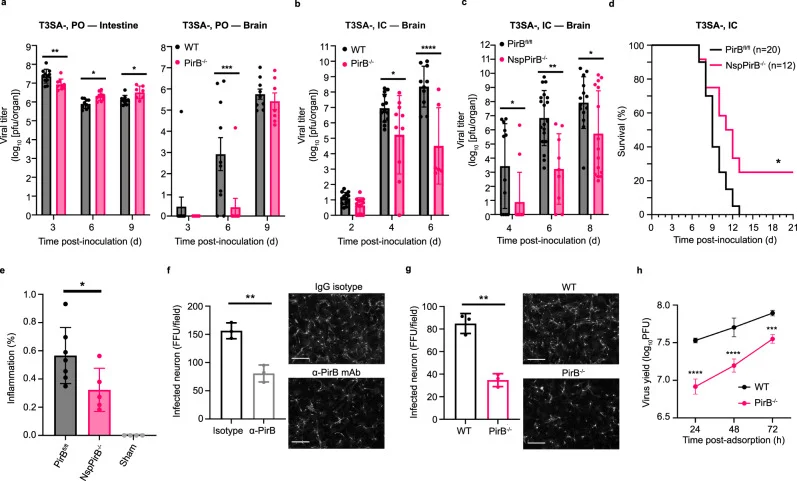
Figure 6 PirB required for efficient replication and pathogenicity of the T3 reovirus in murine CNS
Results
This study, using CRISPR-a screening, identified PirB as a receptor for reovirus infections and demonstrated the probable mechanism. PirB not only serves as an initial binding site, but also mediates intracellular signaling for efficient viral entry. These findings provide a novel perspective for understanding reoviral neuropathogenicity, while offering a new focal point for potential therapeutic strategies against reovirus infections. Future studies may further investigate PirB’s function in different host cells, as well as its mechanisms. Moreover, the damage caused by reovirus infection could be alleviated through the design of new drugs or vaccines targeting the PirB receptor.
Shang, Pengcheng et al. “Paired immunoglobulin-like receptor B is an entry receptor for mammalian orthoreovirus.” Nature communications vol. 14,1 2615. 5 May. 2023, doi:10.1038/s41467-023-38327-6


