ACTN1 Knockout Cells Enable New Insights: α-Actinin-1 Loss Drives Pathology in Megakaryocytes


ACTN1 Knockout Cells Enable New Insights: Loss of α-Actinin-1 in Megakaryocytes Triggers Cascading Pathological Consequences
α-Actinin-1, a member of the α-actinin protein family, has been increasingly implicated in inherited macrothrombocytopenia. However, its precise role in thrombopoiesis and platelet function—as well as the underlying mechanisms—has remained incompletely defined.
On January 16, 2025, the MPN subgroup led by Dr. Jiansong Huang at the Department of Hematology, The First Affiliated Hospital of Zhejiang University, published a study in Blood Advances titled “α-Actinin-1 deficiency in megakaryocytes causes low platelet count, platelet dysfunction, and mitochondrial impairment.” The ACTN1 knockout 293T cell line provided by Ubigene served as a critical cellular model to dissect the function of α-actinin-1 in platelet biogenesis and platelet physiology. The research demonstrated that megakaryocyte-specific deletion of α-actinin-1 in mice led to markedly reduced platelet counts accompanied by enlarged platelet size. Moreover, the investigators uncovered significant defects in platelet function, thrombus formation, and mitochondrial bioenergetics, revealing a cascade of pathological alterations driven by α-actinin-1 deficiency.
Research Background
1. Core Regulatory Basis of Platelet Biogenesis and Function
Platelets, essential for physiological processes such as hemostasis and thrombosis, are produced by mature megakaryocytes. Their biogenesis (megakaryopoiesis) involves multiple highly coordinated steps, in which cytoskeletal remodeling and mitochondrial energy metabolism serve as central regulatory mechanisms for both platelet production and functional maintenance. Previous studies have established that the actin cytoskeleton, actin-binding proteins, and mitochondria-associated factors play critical pathophysiological roles in megakaryocyte maturation, platelet biogenesis, and the preservation of platelet function.
2. Known Functions of α-Actinin-1 and Existing Research Gaps
α-Actinin-1, a member of the actin crosslinking protein family, is widely expressed across multiple tissues. It regulates cytoskeletal dynamics by crosslinking actin filaments into bundled structures and by serving as a scaffold connecting actin to integrins. Previous studies have also suggested that α-actinin-1 helps maintain the inactive conformation of integrin αIIbβ3 in resting platelets. However, the precise role and underlying mechanisms of α-actinin-1 in megakaryopoiesis, platelet biogenesis, and platelet function remain poorly defined. Notably, there is a lack of phenotypic data from α-actinin-1–deficient animal models, such as megakaryocyte-specific knockout mice, which limits our ability to fully elucidate its physiological functions in vivo.
Research Findings
By generating a megakaryocyte/platelet lineage–specific α-actinin-1 conditional knockout mouse model, the research team demonstrated—through complete blood counts and transmission electron microscopy—that loss of α-actinin-1 leads to a reduced platelet count accompanied by increased platelet size in peripheral blood.
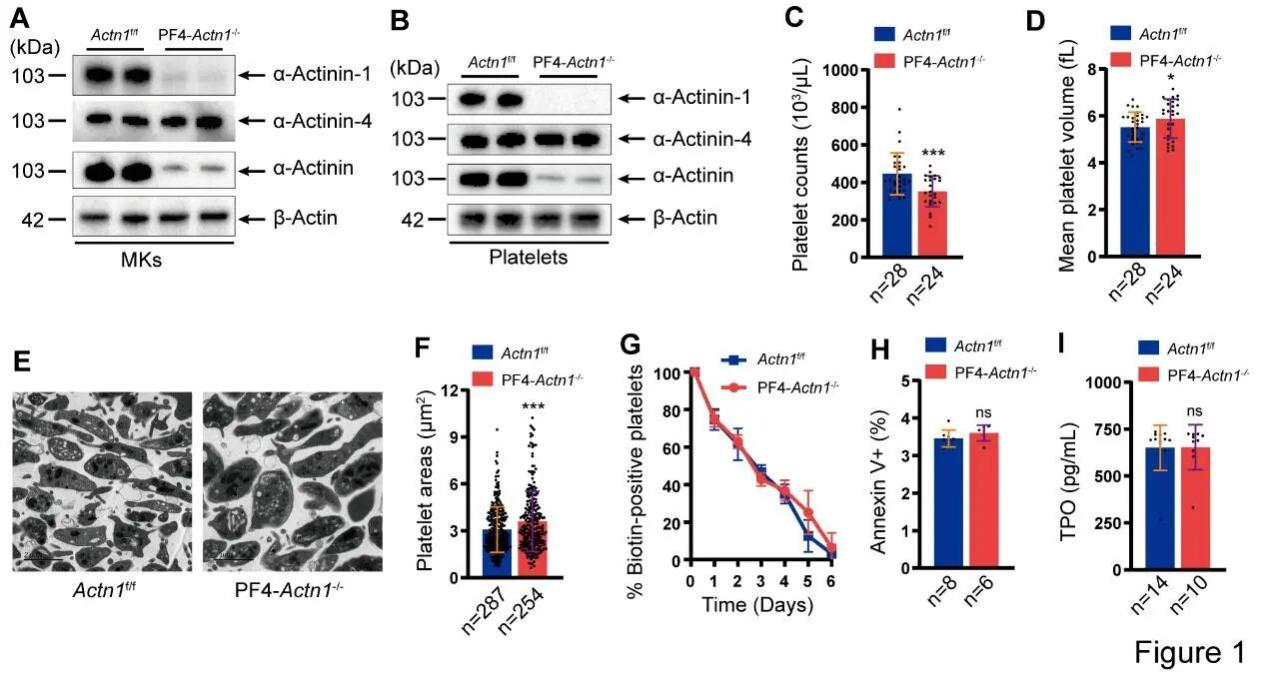
Figure 1. Reduced Platelet Count and Increased Platelet Size in Conditional Knockout Mice
The study demonstrated that the decrease in platelet count observed in megakaryocyte/platelet lineage–specific α-actinin-1 knockout mice results from impaired platelet production, rather than enhanced platelet clearance or alterations in TPO (thrombopoietin) levels. Furthermore, the researchers confirmed that high-level megakaryocyte polyploidization is suppressed in the absence of α-actinin-1. In vitro assays revealed that α-actinin-1–deficient megakaryocytes exhibit impaired colony-forming capacity, reduced migratory ability, and defective proplatelet formation.
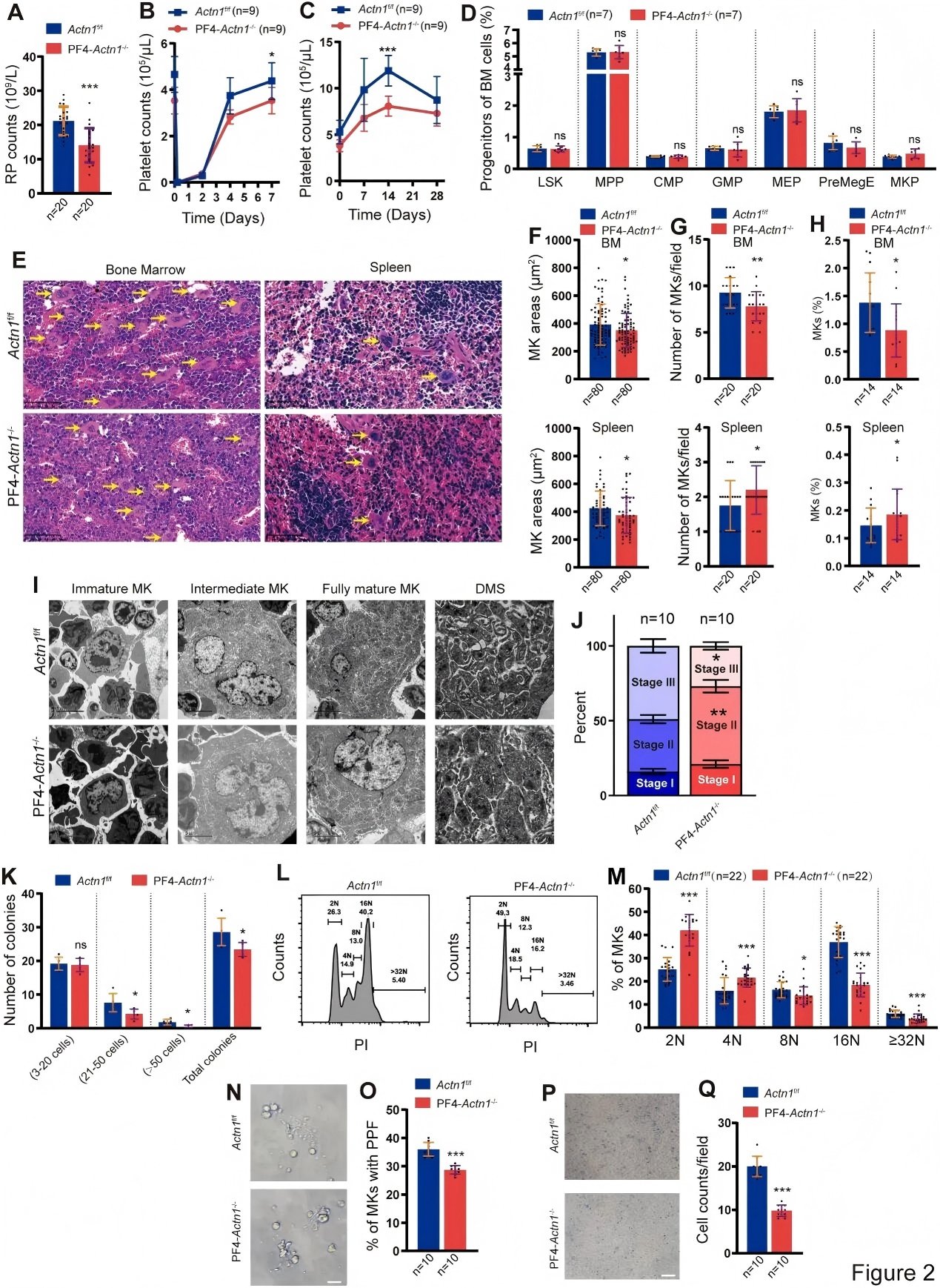
Figure 2. Impaired Colony Formation, Migration, and Proplatelet Formation in α-Actinin-1–Deficient Megakaryocytes
The research team subsequently employed multiple in vivo thrombosis models to assess the thrombus-forming capacity of platelets from the knockout mice. Their findings demonstrated that loss of α-actinin-1 significantly suppresses arterial thrombus formation across several thrombosis models. Mechanistically, α-actinin-1 deficiency was shown to impair a wide spectrum of platelet functions, including adhesion, clot retraction, integrin αIIbβ3 activation, granule secretion, and platelet aggregation, thereby confirming its essential role in platelet-mediated thrombus formation.
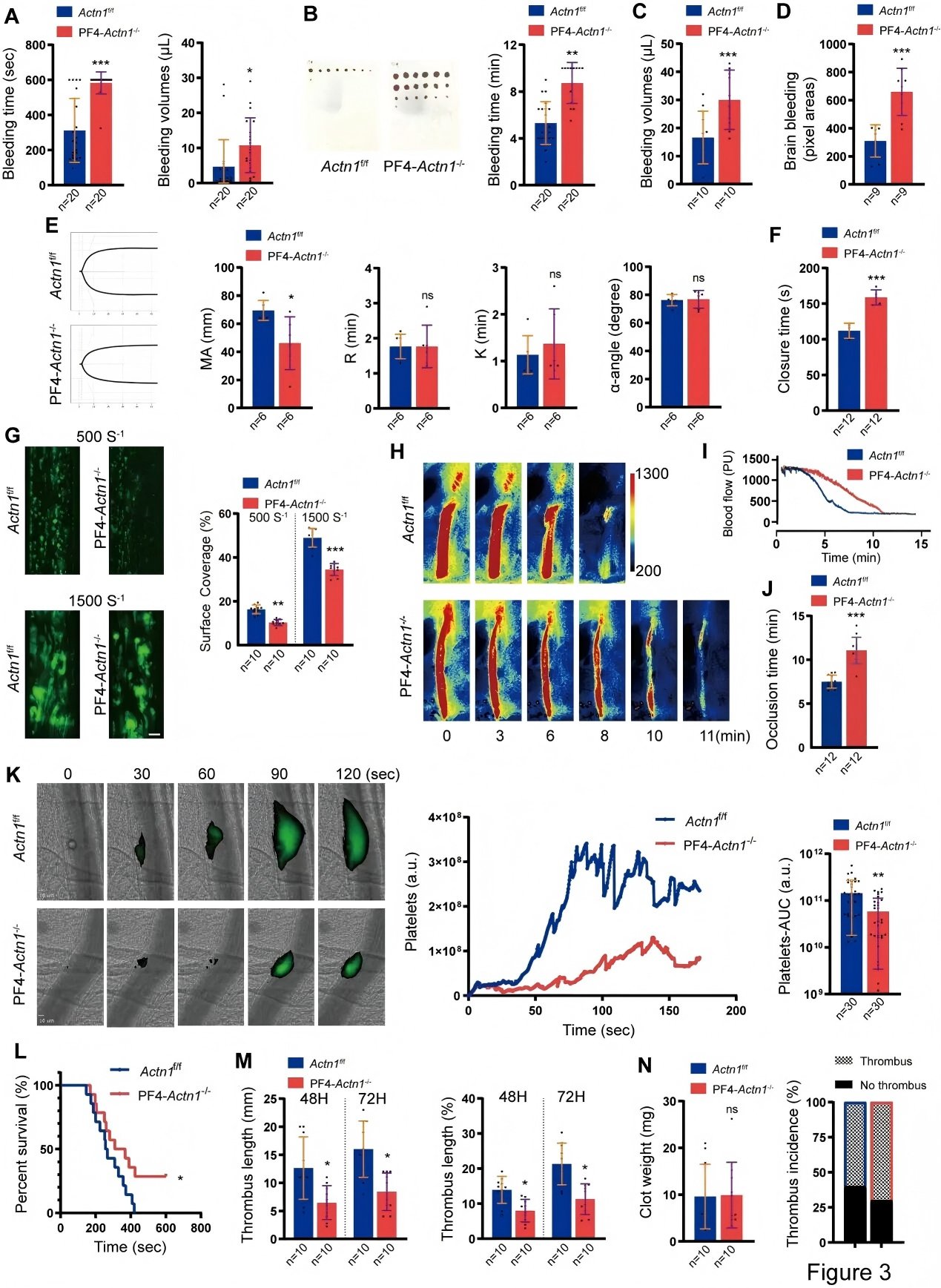
Figure 3. PF4-Actn1⁻/⁻ Mice Exhibit Impaired Primary Hemostasis and Defective Thrombus Formation
Using proteomics, Seahorse metabolic assays, and transmission electron microscopy, the research team further demonstrated that loss of α-actinin-1 suppresses mitochondrial energy metabolism in platelets, accompanied by reduced calcium flux and diminished ROS production. These findings reveal a critical role for α-actinin-1 in maintaining mitochondrial bioenergetic function necessary for normal platelet activation.
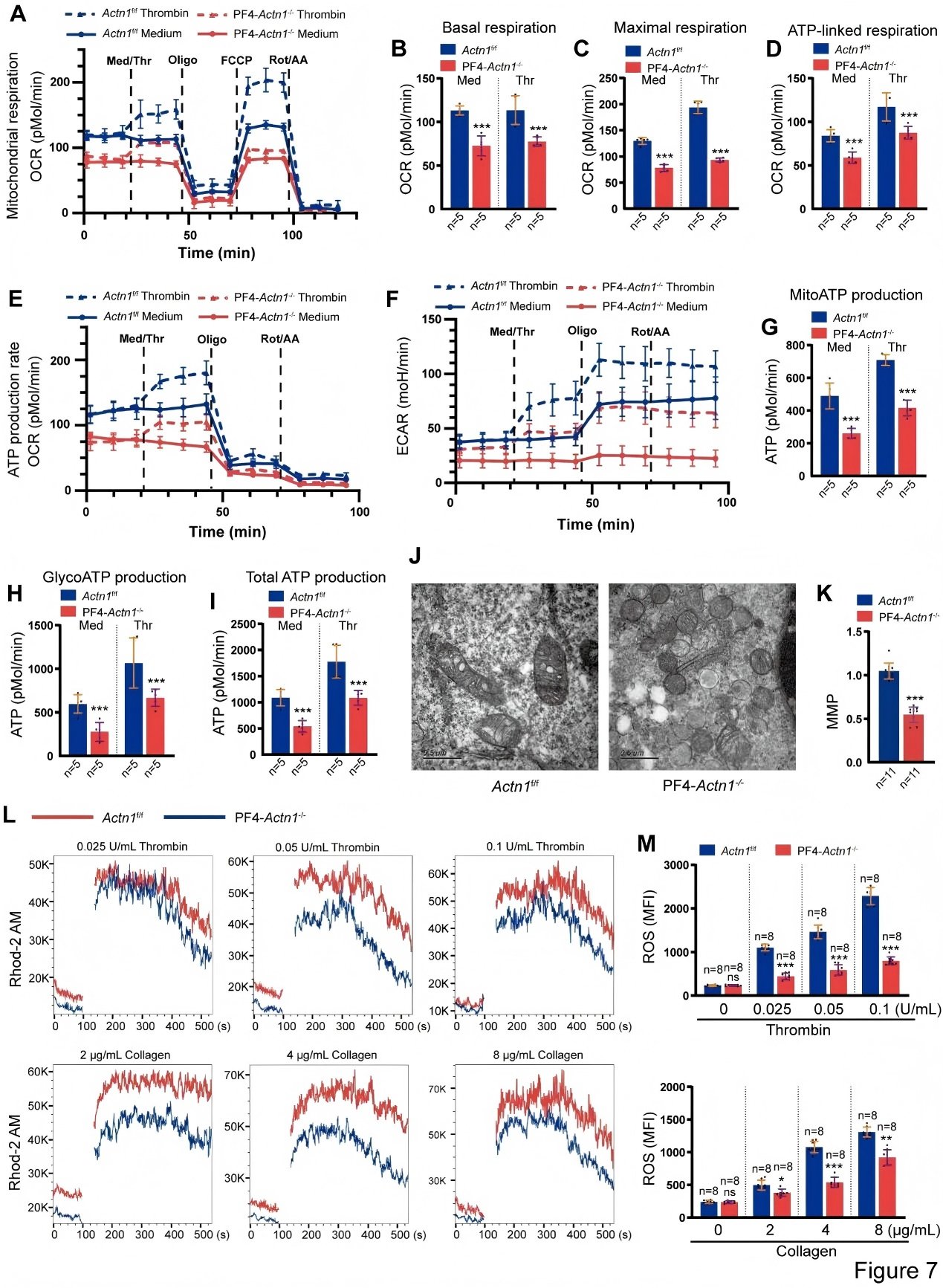
Figure 7. Loss of α-Actinin-1 Impairs Mitochondrial Bioenergetic Function
Conclusions
In this study, the authors generated a megakaryocyte-specific α-actinin-1 knockout mouse model to investigate the role of α-actinin-1 in platelet biogenesis and function. They demonstrated that megakaryocyte-specific deletion of α-actinin-1 leads to reduced platelet counts with increased platelet size, impaired platelet function, defective thrombus formation, and compromised mitochondrial bioenergetic capacity. These findings highlight the critical role of α-actinin-1 in coordinating platelet production, function, and mitochondrial metabolism.
Support Provided by Ubigene
The ACTN1 knockout 293T cell line, the core cellular model used in this study, was provided and technically supported by Ubigene, enabling the research team to uncover the critical role of α-actinin-1 in platelet biogenesis and function.
With extensive practical experience in gene editing and a mature, reliable experimental platform, Ubigene has successfully generated over 8,000 knockout cell lines across more than 300 cell types, covering a wide range of popular research targets.
Researchers can now order KO cell lines starting from $990, with delivery in as fast as 1 week, efficiently accelerating scientific progress.
Explore the full-range Red cotton OmniCell bank to find the cell lines you need. If a specific cell line is not available, customized generation services are also offered to meet the demands of cutting-edge research.
Reference
Lin X, Gao H, Xin M, Huang J, Li X, Zhou Y, Lv K, Huang X, Wang J, Zhou Y, Cui D, Fang C, Wu L, Shi X, Ma Z, Qian Y, Tong H, Dai J, Jin J, Huang J. α-Actinin-1 deficiency in megakaryocytes causes low platelet count, platelet dysfunction, and mitochondrial impairment. Blood Adv. 2025 Mar 11;9(5):1185-1201. doi: 10.1182/bloodadvances.2024014805. PMID: 39813624; PMCID: PMC11925533.



