iPS Cell–Derived EHM for Cardiac Transplantation


Precise Differentiation! iPS Cell–Derived EHM for Cardiac Transplantation, Advancing Regenerative Therapy for Heart Failure
Heart failure remains a major challenge in the cardiovascular field worldwide, with current therapeutic approaches unable to achieve regeneration of damaged myocardium. In March 2025, a study titled “Engineered heart muscle allografts for heart repair in primates and humans” was published in Nature.This research focused on engineered heart muscle (EHM) allografts derived from iPS cells. The efficacy and safety of these allografts were validated across multiple models:
● EHM from both rhesus monkeys and humans exhibited consistent key characteristics with no residual pluripotent stem cells.
● In nude mice as well as healthy and heart failure rhesus monkey models, EHM significantly improved cardiac function, demonstrated long-term engraftment, and established defined safe doses and immunosuppression regimens.
● In patients with advanced heart failure, EHM engrafted successfully, promoted myocardial regeneration, and stabilized patient condition without severe adverse events.
This study provides a complete evidence chain supporting the clinical translation of EHM, addressing the central challenge of myocardial regeneration, and advancing regenerative therapies for late-stage heart failure toward clinical application, laying the foundation for subsequent trials.

I.Research Background
Cardiomyocyte transplantation is a potential strategy for repairing failing hearts, but it has long faced three major challenges:
1. Imbalance between cell retention and therapeutic efficacy: Existing approaches struggle to achieve long-term engraftment of cardiomyocytes, preventing sustained therapeutic effects and limiting improvements in cardiac structure and function.
2. Prominent safety risks: Direct implantation of cardiomyocytes can induce severe adverse effects, including arrhythmias and tumorigenesis, which restrict clinical applicability.
3. Limitations in animal model translation: In xenotransplantation studies using small animals (e.g., rodents), strong immune responses prevent long-term graft survival (no study has demonstrated survival beyond three months in immunocompetent animals) and fail to reliably predict outcomes in large animals or humans.Autologous transplantation, on the other hand, is technically complex and difficult to scale.
Based on these challenges, the research team formulated the central hypothesis: whether engineered heart muscle (EHM) allografts, constructed from iPS cell–derived cardiomyocytes and supporting stromal cells, can achieve structural and functional regeneration in chronic heart failure without severe adverse effects, thereby facilitating the advancement of human clinical trials for heart failure.
II.Research Results
1. Comparative Characterization of Rhesus Monkey and Human EHM: Establishing a Preclinical Translational Model
To determine whether rhesus monkey EHM could serve as a reliable model for the clinical translation of human EHM, the research team generated EHM from rhesus monkey iPS cells and from human GMP-grade iPS cells. Using flow cytometry, single-nucleus RNA sequencing (snRNA-seq), and contractile function assays, they systematically compared the two EHMs in terms of cellular composition, structural organization, and contractile performance. The results demonstrated high concordance between the two EHMs across key metrics:
● Cellular composition: The proportion of ACTN2⁺ cardiomyocytes was 69 ± 3% in rhesus EHM and 81 ± 3% in human EHM.
● Contractile function: Both EHMs generated stable contractions under electrical stimulation.
● Structural features: Sarcomere organization was regular and well-aligned in both models.
Importantly, snRNA-seq revealed no residual pluripotent stem cells in either EHM, effectively eliminating the risk of tumorigenesis. These findings provide a critical foundation for using rhesus monkeys as a preclinical model to assess the safety and efficacy of EHM.
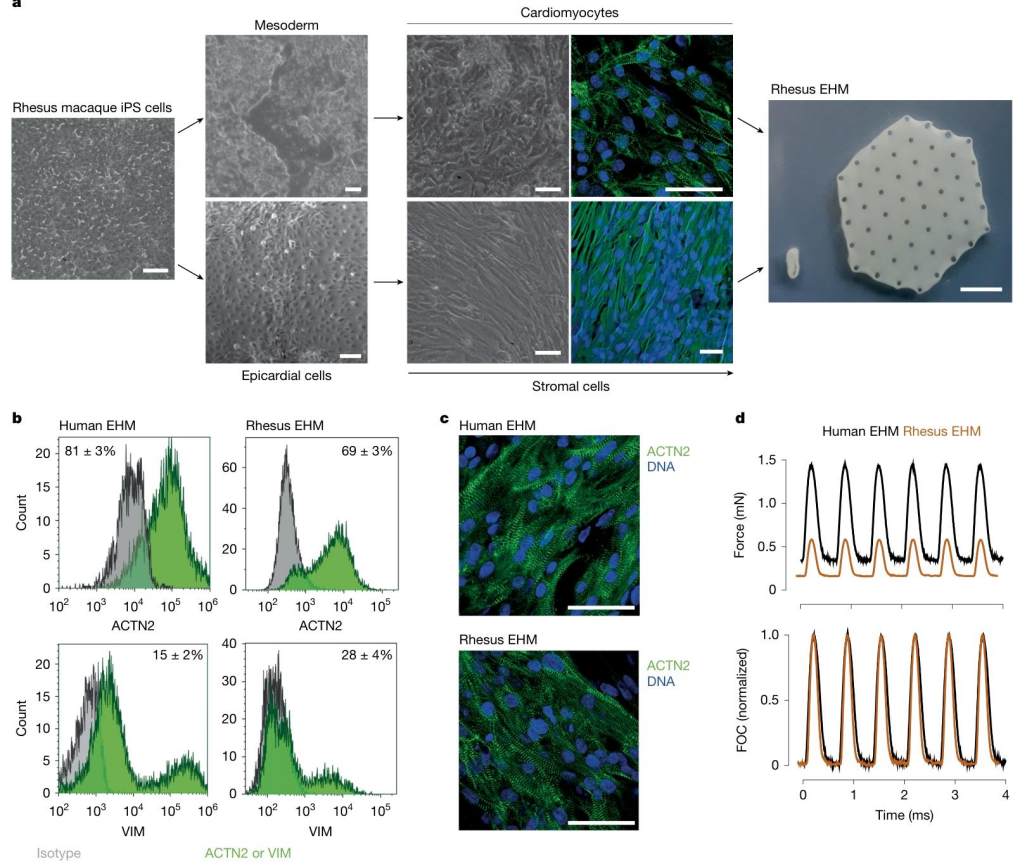
Figure 1. Preparation and Characterization of Rhesus Monkey Engineered Heart Muscle (EHM)
2. Preliminary Exploration in Nude Mouse Model: Assessing EHM Functional Improvement
After confirming the consistency between rhesus monkey and human EHM, the research team further evaluated the feasibility and functional benefits of EHM in a nude mouse ischemia-reperfusion (I/R) injury model. Four days after I/R-induced cardiac injury, 15 mice were randomly assigned to two groups: implantation of viable rhesus EHM (n=7) or lethally irradiated rhesus EHM (n = 8). Cardiac function was assessed 4 weeks post-implantation using echocardiography. Mice receiving viable EHM showed a significant improvement in cardiac function: ejection fraction increased from 47 ± 4% at baseline to 59 ± 3% (P=0.0174), and stroke volume increased from 102 ± 10 μL to 173 ± 13 μL (P=0.0016).In contrast, mice implanted with lethally irradiated EHM displayed no significant functional improvement.
Histological analysis revealed no residual pluripotent stem cells or tumor formation in the viable EHM group. These results provide preliminary evidence that viable EHM has the potential to improve cardiac function in injured hearts without detectable safety concerns.
3. Healthy Rhesus Monkey Model: Determination of Safe EHM Dose and Immunosuppression Regimen
Building on the positive results from the nude mouse model, the research team advanced to a clinically relevant healthy rhesus monkey model to determine the safe EHM dose, the optimal immunosuppression regimen, and long-term graft retention. Fourteen healthy rhesus monkeys were assigned to two cohorts: Cohort 1 (1× EHM, 40 million cells/kg, 3-month follow-up) and Cohort 2 (5× EHM, 20 million cells/kg, 6-month follow-up). Three immunosuppression regimens were tested: tacrolimus alone, tacrolimus plus methylprednisolone, and cyclosporine plus methylprednisolone. Cardiac wall thickness and graft retention were assessed using MRI and histopathology. The results demonstrated:
● Dose-dependent effect on cardiac wall thickening: The 5× EHM group achieved a target wall thickness increase of 4.5 ± 0.6 mm, significantly higher than 1.4 ± 0.3 mm in the 1× EHM group (P < 0.001).
● Optimal immunosuppression regimen: Tacrolimus (target trough concentration 5–15 ng/mL) combined with methylprednisolone (0.15 mg/kg/day) provided the best outcome, allowing EHM retention for up to 6 months, with controlled graft rejection upon cessation. In contrast, one animal under the cyclosporine regimen exhibited autologous graft rejection.
● Safety profile: No arrhythmias or tumor formation were observed in any of the 14 monkeys. Dose-dependent chondrocyte differentiation occurred in 5 animals but was eliminated by extending metabolic selection to 7 days.
Ultimately, tacrolimus plus methylprednisolone was identified as the optimal immunosuppression regimen, and 5× EHM was established as the maximal safe dose for clinical translation.
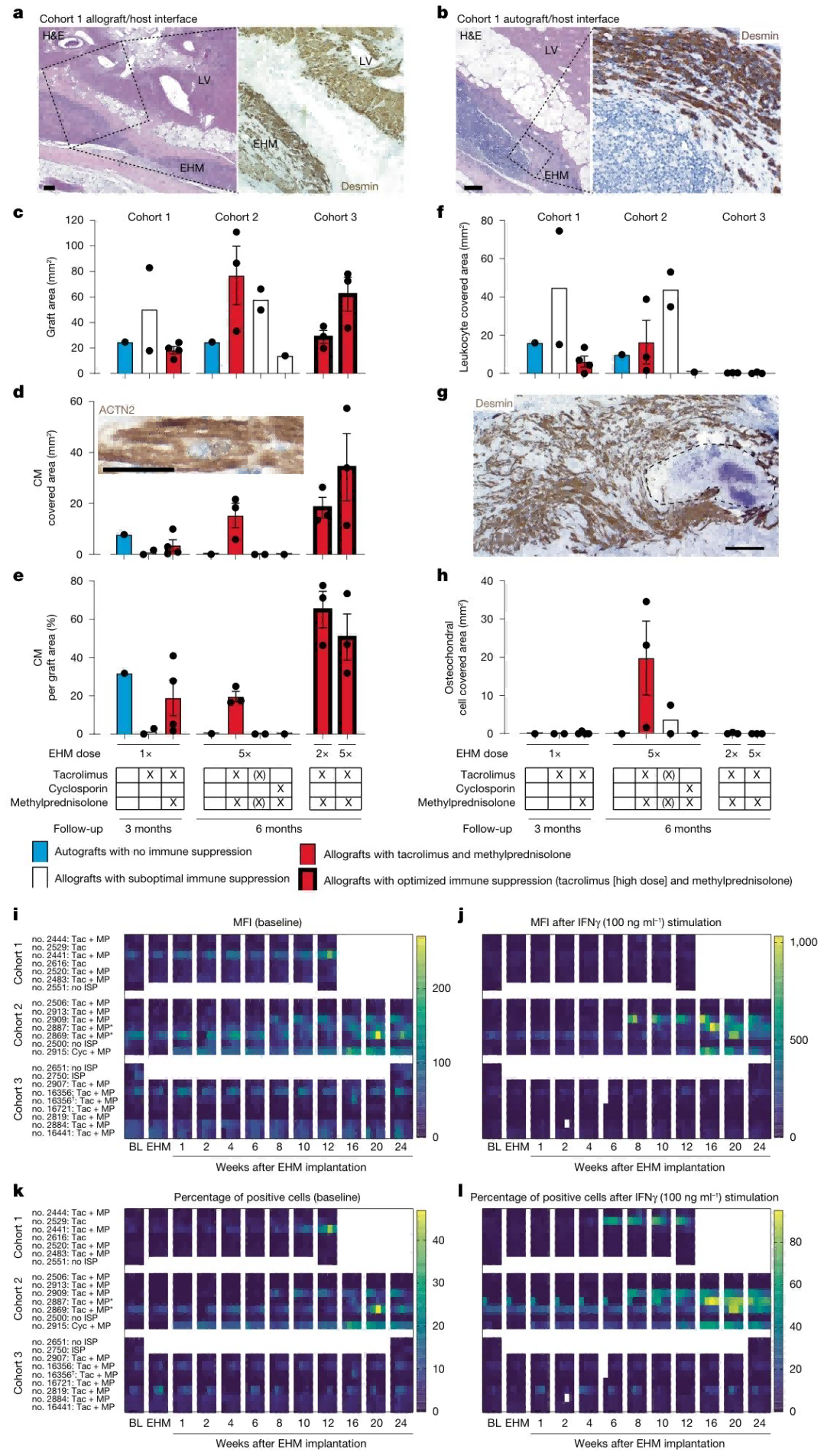
Figure 2. Histopathological Features and Allogeneic Sensitization Responses
4. Chronic Heart Failure Rhesus Monkey Model: Demonstrating EHM-Mediated Repair of Failing Hearts
After establishing the safety parameters of EHM in healthy rhesus monkeys, the research team further evaluated the ability of EHM to restore cardiac structure and function in a pathological model of chronic heart failure. Nineteen rhesus monkeys underwent ischemia-reperfusion (I/R) injury to induce chronic heart failure. Six months later, they were randomly assigned to EHM implantation groups (2× or 5× doses) or control groups (with or without immunosuppression). Over a 6-month follow-up, cardiac wall thickness and ejection fraction were assessed via MRI, while histopathology and MRI were used to evaluate vascularization. The results demonstrated:
● Structural repair: In the 5× EHM group, target wall thickening increased by 2.2 ± 0.1 mm, significantly greater than −0.03 ± 0.07 mm in the control group (P < 0.00001).
● Functional improvement: Among EHM-implanted monkeys, 3/6 showed an increase in wall contraction fraction of 21 ± 0.2%, markedly higher than 0.6 ± 3.3% in controls, and 3/4 showed an increase in ejection fraction of 7 ± 3% (P = 0.0389).
These findings confirm that EHM implantation can promote both structural repair and functional recovery in failing hearts.
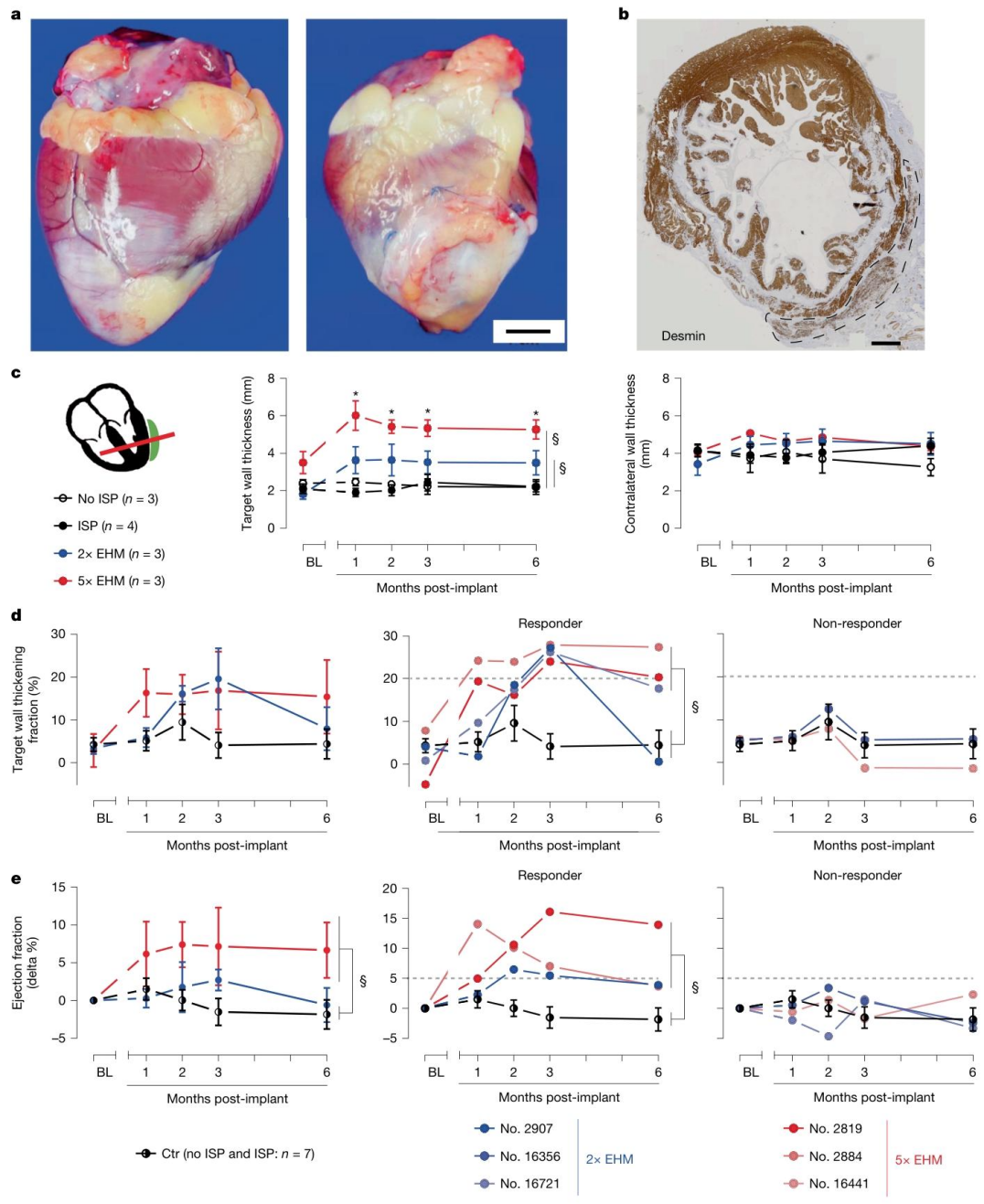
Figure 3. EHM Allografts Promote Local and Global Cardiac Functional Recovery via Myocardial Regeneration in a Chronic Heart Failure Model
Regarding vascularization, gadolinium-enhanced MRI and histopathology confirmed that the capillary density of EHM grafts reached 281 ± 37 capillaries/mm², matching the vascularization level of the host myocardium, thereby supporting long-term graft survival.These findings demonstrate that EHM can not only engraft safely in healthy hearts but also effectively restore the structure and function of failing hearts.
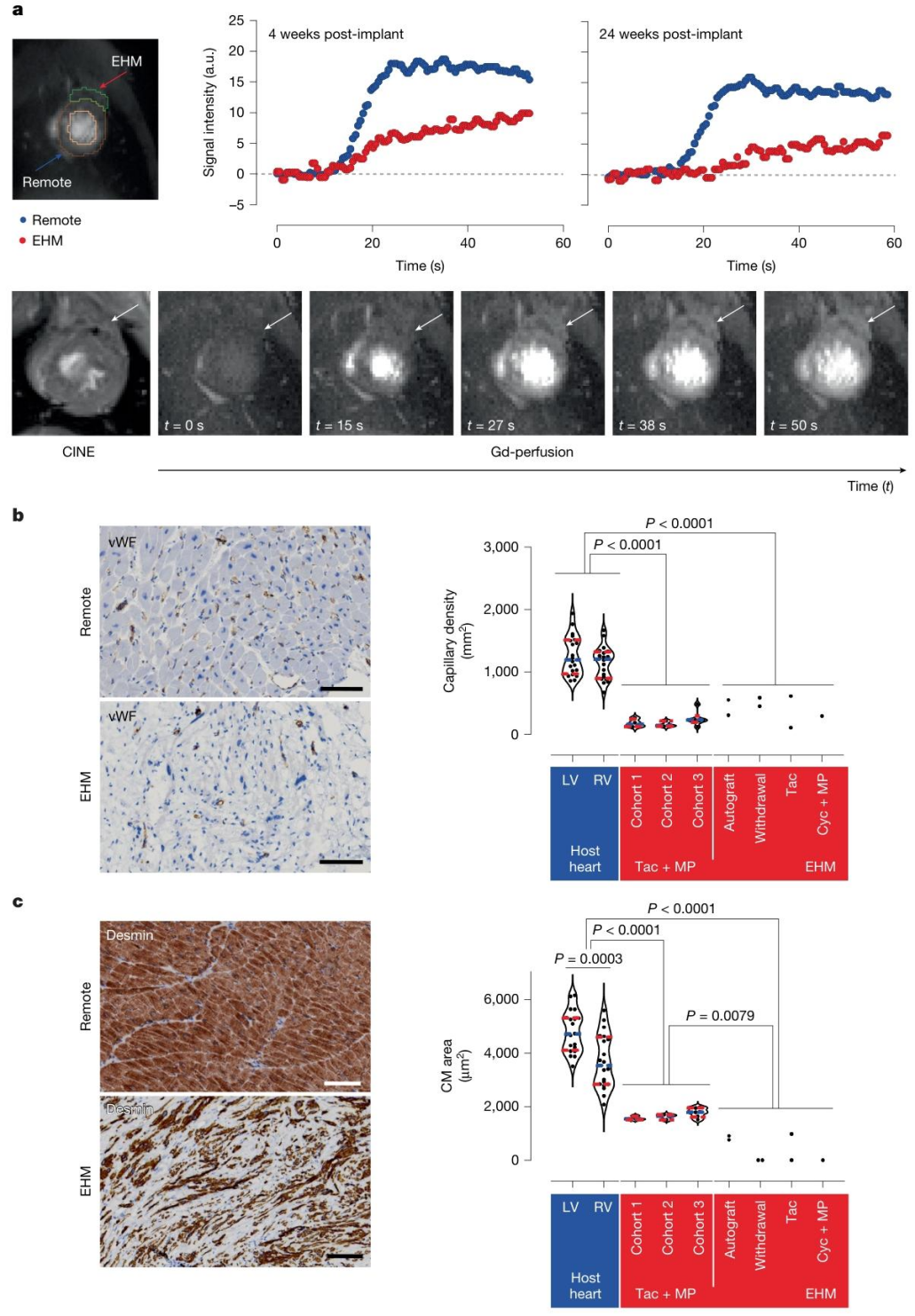
Figure 4. Evidence of Vascularization and Perfusion in EHM Allografts
5. Human Clinical Validation: Achieving the Translational Breakthrough from Animals to Humans
Following extensive validation in multiple animal models, the research team advanced to a human clinical trial to evaluate the feasibility of translating EHM therapy from animals to humans. A single patient with advanced heart failure (NYHA class III, ejection fraction 35%) received implantation of 10× EHM (400 million cells). Three months later, due to disease progression, the patient underwent heart transplantation, allowing the research team to assess graft retention and myocardial regeneration via histopathology and genetic sequencing.
The results demonstrated that the EHM graft successfully engrafted in the patient’s heart.Although the cardiomyocyte area within the graft (947 ± 35 μm²) was smaller than that of host cardiomyocytes (3632 ± 168 μm²), regular sarcomere structures had already formed. Capillary density within the graft reached 187 ± 5 capillaries/mm², indicating effective local vascularization. Postoperatively, the patient remained clinically stable, with ejection fraction improving to 39%. No arrhythmias or other adverse effects were observed, and no donor-specific antibodies were detected.These findings provide the first evidence in humans that EHM can promote myocardial regeneration safely, establishing a critical foundation for future large-scale clinical trials.
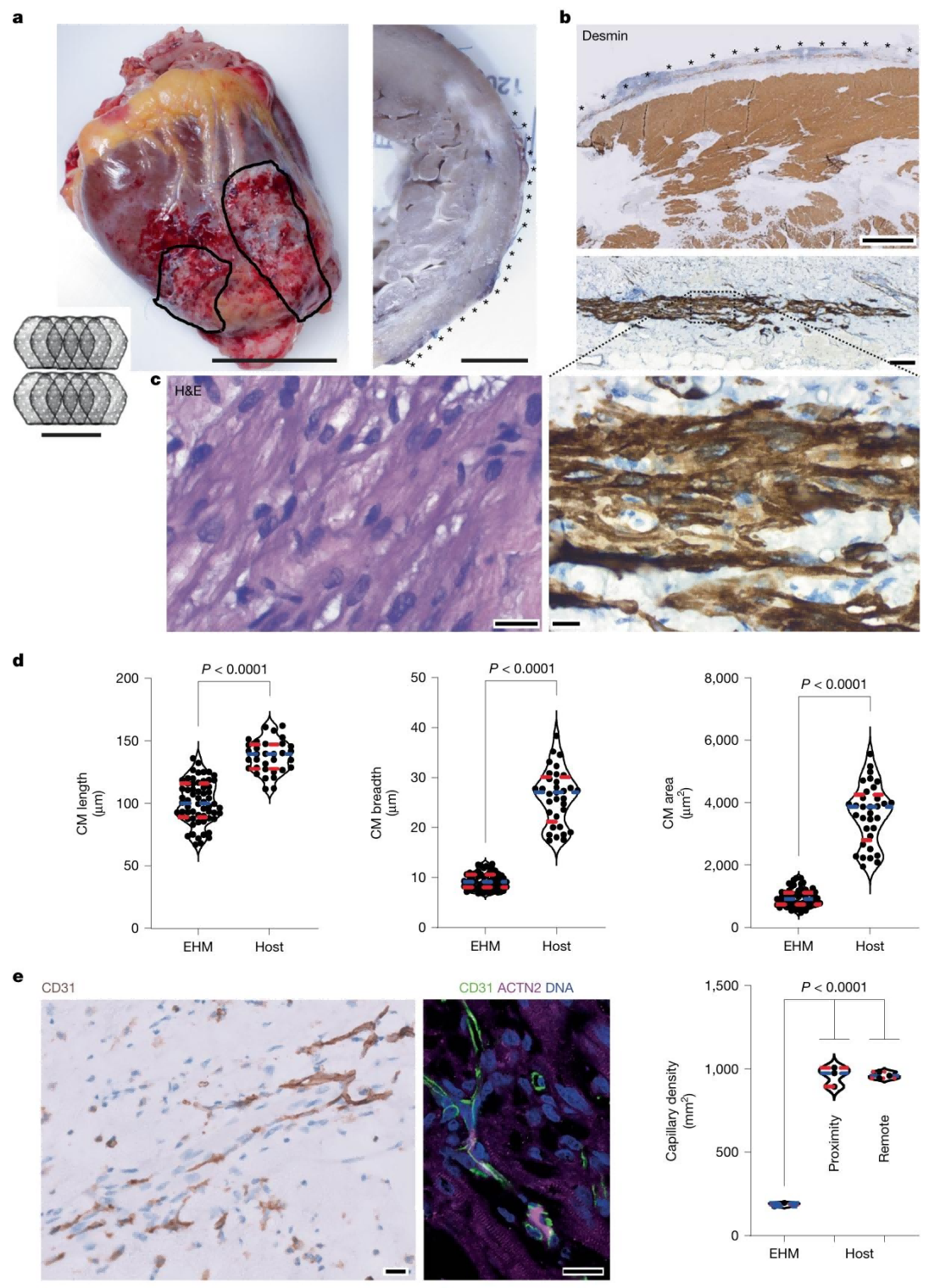
Figure 5. Myocardial Regeneration in the Human Heart
III.Significance of the Study
The significance of this study can be summarized in two major aspects:
First, it establishes the translational value of EHM grafts. Rhesus monkey iPS cell–derived EHM allografts achieved a complete chain of “long-term engraftment (6 months) → structural repair → functional improvement” in a large animal model, without severe safety concerns (no arrhythmias or tumor formation), providing critical experimental evidence for human clinical application.
Second, the study provides the first demonstration of myocardial regeneration by EHM in a patient with advanced heart failure. The patient remained clinically stable without serious adverse events, confirming the feasibility of translation from rhesus monkeys to humans. This directly supports the further advancement of the BioVAT-HF Phase 1/2 clinical trial, opening a new avenue for regenerative therapy in late-stage heart failure.
Overall, this research not only addresses the “engraftment–efficacy–safety” triangle in myocardial regeneration but also establishes a crucial bridge from basic research to clinical application. Looking forward, with the development of technologies such as low-immunogenic EHM and 3D-printed EHM, regenerative therapy for heart failure is poised to move from clinical trials to routine clinical practice, offering new hope to millions of patients worldwide.
Stem cell–based research is accelerating the advancement of human medicine, and an increasing number of “regenerative therapies” are moving toward clinical application. Stem cell–based gene editing also enables deeper insights into the evolutionary dynamics of human cells.
At Ubigene, leveraging years of experience in gene editing, we have successfully performed gene modifications in stem cells. If you are conducting research involving stem cell–based gene editing, we can provide professional stem cell gene editing services.
Contact us to learn more about our offerings.
Reference
Jebran AF, Seidler T, Tiburcy M, Daskalaki M, Kutschka I, Fujita B, Ensminger S, Bremmer F, Moussavi A, Yang H, Qin X, Mißbach S, Drummer C, Baraki H, Boretius S, Hasenauer C, Nette T, Kowallick J, Ritter CO, Lotz J, Didié M, Mietsch M, Meyer T, Kensah G, Krüger D, Sakib MS, Kaurani L, Fischer A, Dressel R, Rodriguez-Polo I, Stauske M, Diecke S, Maetz-Rensing K, Gruber-Dujardin E, Bleyer M, Petersen B, Roos C, Zhang L, Walter L, Kaulfuß S, Yigit G, Wollnik B, Levent E, Roshani B, Stahl-Henning C, Ströbel P, Legler T, Riggert J, Hellenkamp K, Voigt JU,
Hasenfuß G, Hinkel R, Wu JC, Behr R, Zimmermann WH.
Engineered heart muscle allografts for heart repair in primates and humans. Nature. 2025 Mar;639(8054):503-511. doi: 10.1038/s41586-024-08463-0.
Epub 2025 Jan 29. PMID: 39880949; PMCID: PMC11903342.



