HeLa Knockout Cell lines


HeLa Knockout Cell lines: A Classic Model for Functional Genomics
Among all cell lines established in the history of biomedical research, few have had as profound and lasting an impact as the HeLa cell line . Since its isolation in 1952 from the cervical carcinoma of Henrietta Lacks, HeLa has exhibited an unprecedented capacity for unlimited proliferation in vitro, breaking the traditional barrier of finite human cell lifespan. This unique property established HeLa as the first immortalized human cell line, laying the foundation for decades of experimental advances—from the development of the polio vaccine to groundbreaking discoveries in cancer biology and molecular therapeutics.
The emergence of CRISPR/Cas9 genome editing has revolutionized the utility of HeLa cells, enabling precise gene knockout and loss-of-function studies at an unprecedented scale. Such genetically engineered HeLa knockout cell lines provide powerful platforms for dissecting gene function, elucidating pathogenic mechanisms, and accelerating target-based drug discovery. By integrating classical cellular models with modern genome engineering, HeLa knockout systems have become a pivotal bridge linking fundamental research to translational medicine.
Ⅰ.Core Research Value of Gene Knockout HeLa Cell Lines
Since their isolation in 1952, HeLa cells have been regarded as a “universal model” in the life sciences owing to their unique biological properties. The advent of gene editing technologies has brought a qualitative leap in their research value, which can be summarized in three major dimensions:
1. Decoding Gene Function
By knocking out target genes in HeLa cells, researchers can systematically compare phenotypic changes—such as alterations in cell morphology, proliferation rate, and signaling pathways—to elucidate the biological roles of specific genes. For instance, the knockout of genes involved in DNA damage repair vividly reveals cellular defects in response to genomic stress, providing critical insights into the molecular mechanisms underlying fundamental life processes.
2. Elucidating Disease Mechanisms
In cancer research, HeLa knockout models can mimic genetic abnormalities that drive tumorigenesis, thereby uncovering the intrinsic logic of malignant proliferation, invasion, and metastasis. In infectious disease studies, disruption of genes essential for viral entry helps identify the key receptors and signaling pathways exploited by pathogens. During the COVID-19 pandemic, for example, HeLa-based gene knockout assays played a pivotal role in rapidly confirming the receptor used by SARS-CoV-2 to enter human cells, accelerating the pace of fundamental research.
3. Accelerating Drug Discovery and Development
Gene knockout HeLa cell lines serve as efficient platforms for target-based drug screening and mechanistic validation. By constructing disease-relevant knockout models, researchers can identify potential therapeutic compounds, assess their efficacy and safety, and differentiate on-target effects from off-target or resistance mechanisms. Comparative analysis between wild-type and knockout HeLa cells offers a powerful strategy to clarify drug–target interactions, thereby providing robust scientific evidence for clinical pharmacology and precision medicine.
Ⅱ.Case Studies: Applications of Gene Knockout HeLa Cells in Research
Ubigene has successfully developed customized gene knockout HeLa cell lines for multiple research teams, enabling significant breakthroughs across a range of scientific projects. The following two representative cases highlight the broad application value of these models in diverse research fields.
Case 1. IF = 6.9 | Contribution of TBK1 Knockout HeLa Cell Line to the Study of TBK1-Mediated Type I Interferon Response
DOI: 10.1016/j.celrep.2025.116336
Research Background
TANK-binding kinase 1 (TBK1) is a central serine/threonine kinase that initiates the innate antiviral immune response. Aberrant activation of TBK1 leads to excessive production of type I interferons (IFN-I) and triggers pathological inflammation. Itaconic acid (ITA) and its derivatives have been proposed to modulate immune signaling by targeting TBK1; however, the specificity of these compounds and the functional roles of key TBK1 modification sites remained unclear. To address this, Ubigene provided a TBK1 knockout (TBK1 KO) HeLa cell line , which enabled researchers to dissect the role of TBK1 in IFN-I signaling and to elucidate the regulatory mechanism of itaconic acid.
Experimental Design
Using the CRISPR/Cas9 system, researchers generated a TBK1 knockout HeLa cell line, with wild-type (WT) HeLa cells serving as the control. Cells were transfected with either WT or mutant TBK1 plasmids (e.g., C605S) and treated with itaconic acid (ITA) or 4-octyl itaconate (4-OI). A combination of luciferase reporter assays, qPCR, Western blotting, and kinase activity analyses was used to evaluate the role of TBK1 and its post-translational modifications in the IFN-I response.
Results
● Validation of TBK1 as an Essential Regulator of IFN-β Production
In TBK1 KO HeLa cells, transfection with WT-TBK1 restored IFN-β reporter activity and IFNB mRNA expression, whereas untransfected or vector-transfected cells exhibited suppressed IFN-β induction. These findings confirmed that TBK1 is indispensable for the activation of the IFN-I pathway.
● Identification of the Functional Modification Site in TBK1
In TBK1 KO HeLa cells expressing the C605S mutant (cysteine 605 substituted with serine), the itaconate-mediated alkylation of TBK1 was markedly reduced. This led to decreased TBK1 dimerization and phosphorylation, resulting in suppressed IFN-β reporter activity. Notably, the inhibitory effect of itaconic acid on IFN-β production was completely abolished in the C605S mutant, demonstrating that Cys605 is the critical residue for itaconate-mediated alkylation and regulation of TBK1 activity.
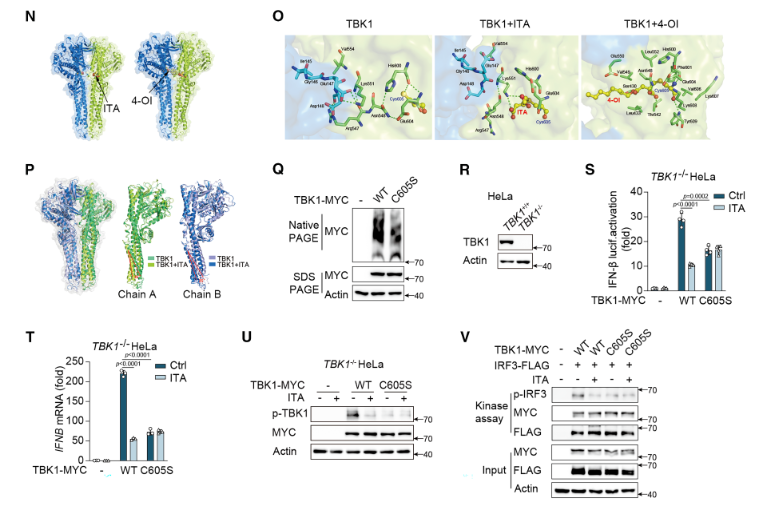
Figure 1. Validation of TBK1 as the Essential Target of Itaconic Acid (ITA) in Regulating the Type I Interferon (IFN-I) Pathway
● Confirmation of Drug Specificity
In TBK1 knockout HeLa cells, treatment with itaconic acid (ITA) or 4-octyl itaconate (4-OI) failed to suppress IFN-β production or TBK1 phosphorylation. However, upon reintroduction of WT-TBK1, the inhibitory effects of the compounds were restored. These results excluded the possibility of nonspecific interference with other signaling pathways and confirmed that ITA and 4-OI exert their immunomodulatory effects specifically through targeting TBK1.
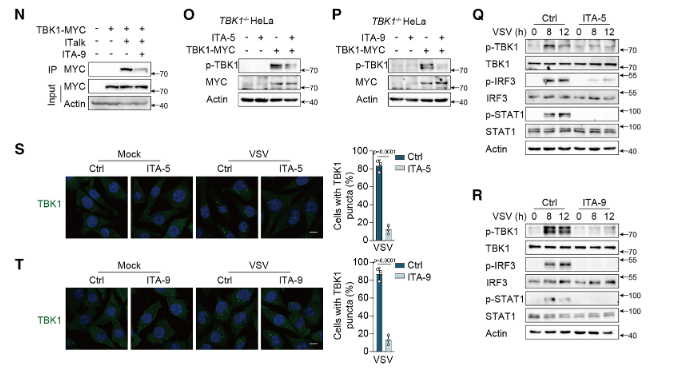
Figure 2. Validation of the Targeted Activity of Novel TBK1 Inhibitors (ITA-5 and ITA-9)
Case 2. IF = 26.8 | Contribution of LDL-R Knockout HeLa Cell Line to the Study of Tumor-Targeting Mechanisms of Peptide Amphiphiles (PAs)
DOI: 10.1002/adma.202509359
Research Background
The peptide amphiphile (PA) SA-E can prolong systemic circulation by binding to serum lipoproteins such as high-density lipoprotein (HDL), thereby enhancing tumor targeting. However, it remains unclear whether its cellular internalization depends on lipoprotein receptors, particularly the low-density lipoprotein receptor (LDLR). To determine whether SA-E uptake occurs through receptor-independent mechanisms and to rule out receptor-mediated endocytosis, researchers required a definitive genetic model. The LDLR knockout (LDLR⁻/⁻) HeLa cell line , developed by Ubigene, provided a critical cellular tool for elucidating the interactions between PAs and endogenous lipoproteins.
Experimental Design
Using the CRISPR/Cas9 genome editing, researchers established an LDLR knockout HeLa cell line, with wild-type (WT) HeLa cells as controls. Cellular uptake was assessed by incubating cells with Cy5-labeled SA-E, followed by quantitative fluorescence microscopy. In parallel, WT and LDLR⁻/⁻ HeLa cells were subcutaneously implanted into nude mice to establish xenograft tumor models. After intravenous injection of ICG-labeled SA-E, in vivo fluorescence imaging (IVIS) was performed to compare tumor accumulation between the two groups.
Results
● LDLR Verification of LDLR–Independent Cellular Uptake of SA-E
The uptake of SA-E in LDLR⁻/⁻ HeLa cells was comparable to that observed in WT HeLa cells, indicating that SA-E internalization does not require LDLR mediation. Instead, SA-E enters cells through interactions with cholesterol-enriched lipid raft domains on the plasma membrane, confirming that its cellular uptake occurs via a receptor-independent mechanism.
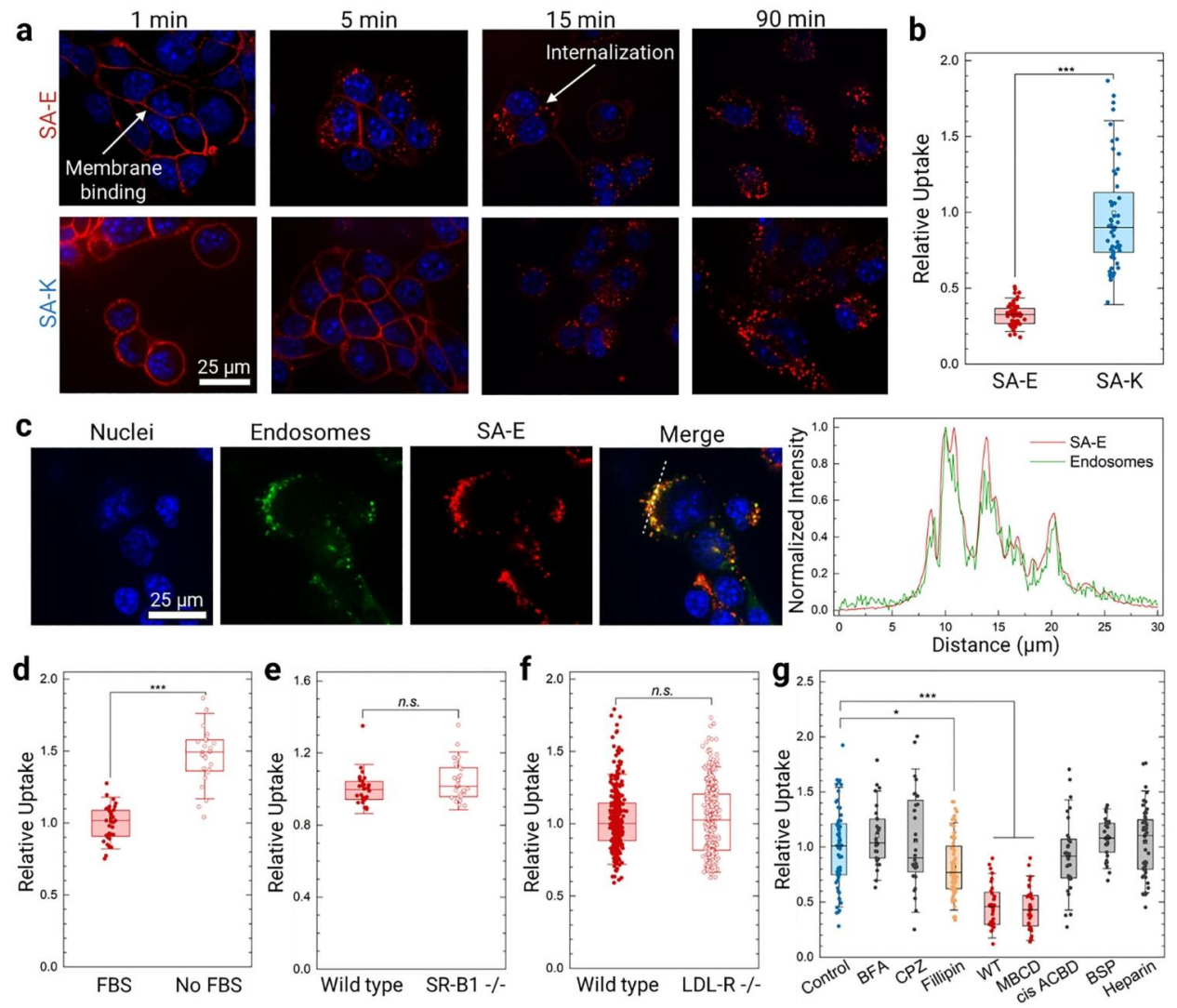
Figure 3. Validation of LDL-R–Independent Cellular Uptake of SA-E
● Tumors derived from LDLR⁻/⁻ HeLa cells exhibited SA-E accumulation levels comparable to those formed by WT HeLa cells, both showing strong fluorescence signals. This demonstrates that SA-E can efficiently target tumors even in the absence of LDLR, supporting a universal targeting mechanism mediated by lipoprotein “hitchhiking” and plasma membrane lipid raft interactions.
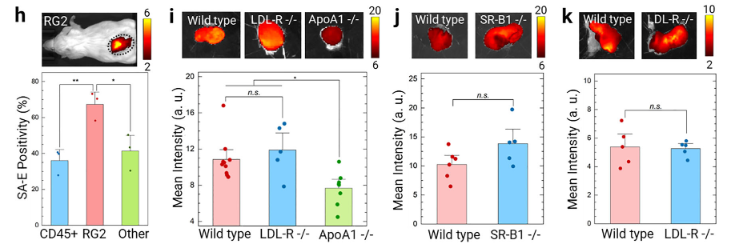
Figure 4. Validation that SA-E Tumor Targeting Is Independent of Tumor Cell LDLR Expression
● Support for Broad-Spectrum Application Potential
These results, consistent with experiments using SR-B1 knockout cells and lipoprotein receptor–deficient mice, collectively demonstrate that SA-E tumor targeting does not depend on specific lipoprotein receptors. This provides critical evidence supporting its potential use across solid tumors with varying receptor expression profiles.
Ⅲ.Ubigene Gene Knockout Cell Services
Ubigene has extensive experience in the field of genome editing and has accumulated a wealth of technical expertise over the years. To date, the company has successfully generated over 8,000 gene knockout cell lines , spanning more than 300 cell types, including tumor cells, immortalized cell lines, and iPS cells.
Leveraging the proprietary Red Cotton CRISPR Gene Editing Designer, Ubigene offers 3 standard knockout strategies, which can be customized to meet specific research objectives. In addition, three complementary delivery methods—RNP-based, plasmid-based, and viral-based editing—are available, allowing researchers to select the optimal approach for their experimental needs.
Related Hela KO Cell Line Products
Reference
[1]Pittner NA, McCoy JR, Bui D-C, McBride JW. TRP75-mediated STAT3 activation promotes anti-apoptotic signaling and Ehrlichia chaffeensis infection. Infect Immun. 2025 Nov 11;93(11):e0045925. doi: 10.1128/iai.00459-25. Epub 2025 Oct 20. PMID: 41115066.
[2]Chai L, Li C, Wang X, Qin Y, Sun H, Du J, Yang L, Hu D, Xiong J, Zhao Z, Gong R, Wu T, Wu M, Nie M, Gao J, Jia J, Gao C, Zhao W, Zhou H, Kang D, Jia M. IRG1 catalyzed energy metabolite itaconic acid restrains type I interferon-dependent immune responses by alkylation of TBK1. Cell Rep. 2025 Oct 28;44(10):116336. doi: 10.1016/j.celrep.2025.116336. Epub 2025 Sep 26. PMID: 41014555.
[3]Xiang L, Stewart MR, Mooney K, Dai M, Drennan S, Holland S, Quentel A, Sabuncu S, Kingston BR, J Dengos I, Bonic K, Goncalves F, Yi X, Henderson MI, Ranganathan S, Branchaud BP, Muldoon LL, Jr RFB, Fischer JM, Yildirim A. Peptide Amphiphiles Hitchhike on Endogenous Biomolecules for Enhanced Cancer Imaging and Therapy. Adv Mater. 2025 Oct 4:e09359. doi: 10.1002/adma.202509359. Epub ahead of print. PMID: 41045193.



