Breakthrough in the Cell Series: Ubigene Helps Elucidate a Novel α-Ketoglutarate–Driven Anti-Aging Mechanism in Mesenc


Breakthrough in the Cell Series: Ubigene Helps Elucidate a Novel α-Ketoglutarate–Driven Anti-Aging Mechanism in Mesenchymal Stem Cells

In July 2025, researchers from the China Academy of Chinese Medical Sciences and collaborating institutions published a significant study in Cell Reports, a journal of Cell Press.In this work, Ubigene provided the research team with critical cell models, playing an essential role in facilitating the project’s smooth progress. The study, titled “Identifying the Target, Mechanism, and Agonist of α-Ketoglutaric Acid in Delaying Mesenchymal Stem Cell Senescence”, uncovers a novel mechanism by which α-ketoglutaric acid (AKG) delays the senescence of mesenchymal stem cells (MSCs). Specifically, AKG promotes hydroxylation of ribosomal protein S23 (RPS23) and enhances translational fidelity, thereby slowing MSC aging.The researchers further demonstrated that AKG generated via isocitrate dehydrogenase 1 (IDH1) is critical for this process. Moreover, activating this pathway with scutellarin (Scu) alleviated aging phenotypes in mice. This pivotal work offers fresh insights and new directions for the development of anti-aging strategies.

Highlights
- 1.AKG delays MSC senescence by enhancing hydroxylation of RPS23 via the iron-dependent dioxygenase OGFOD1.
- 2.Reduced IDH1 activity lowers AKG levels and drives MSC senescence; reactivation of IDH1 can reverse this process.
- 3.Scutellarin (Scu) activates IDH1, elevates AKG levels, and mitigates age-related phenotypes in mice.
- 4.AKG improves translational fidelity, maintaining a balance between protein synthesis and accuracy.
Background
α-Ketoglutarate (AKG) is a key metabolite involved in multiple metabolic and cellular pathways. Previous studies have shown that AKG can delay aging, and it is considered a safe dietary supplement that may extend healthy lifespan and potentially reduce disease incidence. Isocitrate dehydrogenase 1 (IDH1) is a critical enzyme for AKG biosynthesis and has been linked to age-related diseases in humans. Although indirect evidence from multiple studies has suggested the importance of the IDH1–AKG signaling axis in aging, its precise molecular mechanism and anti-aging targets remain unclear.
Stem cell senescence is recognized as both a hallmark and a driving force of organismal aging. Thus, exploring strategies to increase stem cell abundance as a means of delaying aging has become an active area of research. Loss of protein homeostasis is frequently observed during aging. Mechanistic defects or mutations can compromise translational fidelity, resulting in aberrant protein folding. This, in turn, leads to the production of dysfunctional proteins and the accumulation of harmful protein aggregates. Therefore, identifying anti-aging molecules or molecular combinations that can simultaneously enhance protein synthesis rates and improve translational accuracy represents a promising approach to counteracting stem cell senescence. In this study, the authors aimed to investigate the potential role and molecular mechanisms of IDH1 and AKG in regulating protein homeostasis during mesenchymal stem cell (MSC) senescence.
Results
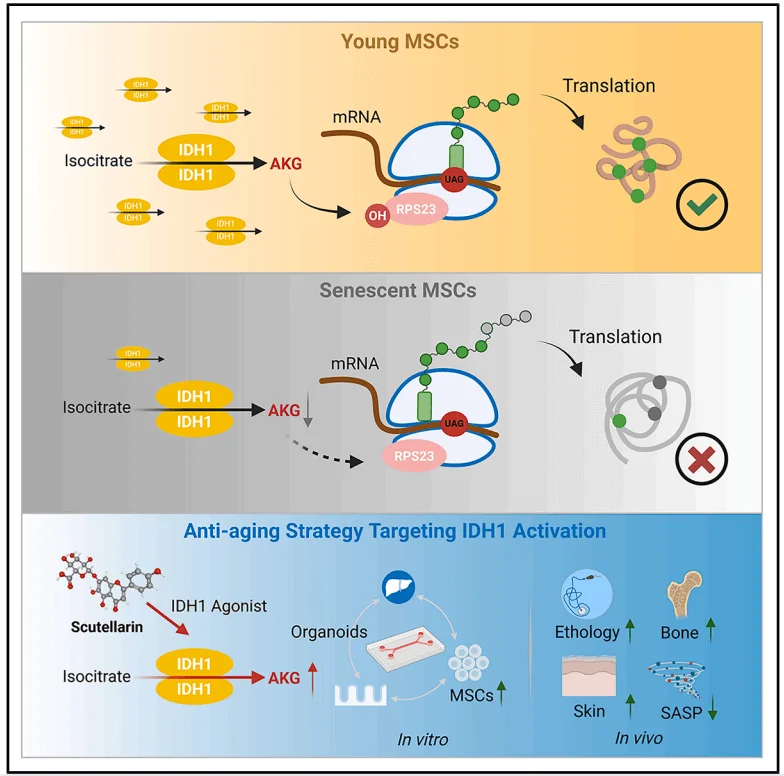
The tricarboxylic acid (TCA) cycle serves as a central hub integrating metabolic and signaling pathways within the aging network, with α-ketoglutarate (AKG) as one of its key intermediates. To examine metabolic alterations in the TCA cycle during mesenchymal stem cell (MSC) senescence, the researchers employed ^13C_6-labeled glucose to trace the generation of related metabolites. Compared with early-passage MSCs, late-passage MSCs exhibited significantly reduced production of citrate, aconitate, AKG, fumarate, malate, and acetyl-CoA.Cell proliferation assays revealed that among the metabolites tested, only AKG and its derivative, dimethyl α-ketoglutarate (DM-AKG), markedly enhanced MSC proliferation. Further analysis of MSCs undergoing either physiological senescence or D-galactose (D-Gal)-induced pathological replicative senescence showed that AKG levels were significantly lower in both senescent conditions compared with normal MSCs.Subsequent supplementation experiments demonstrated that both AKG and DM-AKG increased intracellular AKG concentrations, with DM-AKG showing pronounced effects at relatively low doses.Collectively, these findings suggest that AKG may serve as a metabolic biomarker of MSC senescence and that reduced AKG production could contribute to the onset of MSC aging.
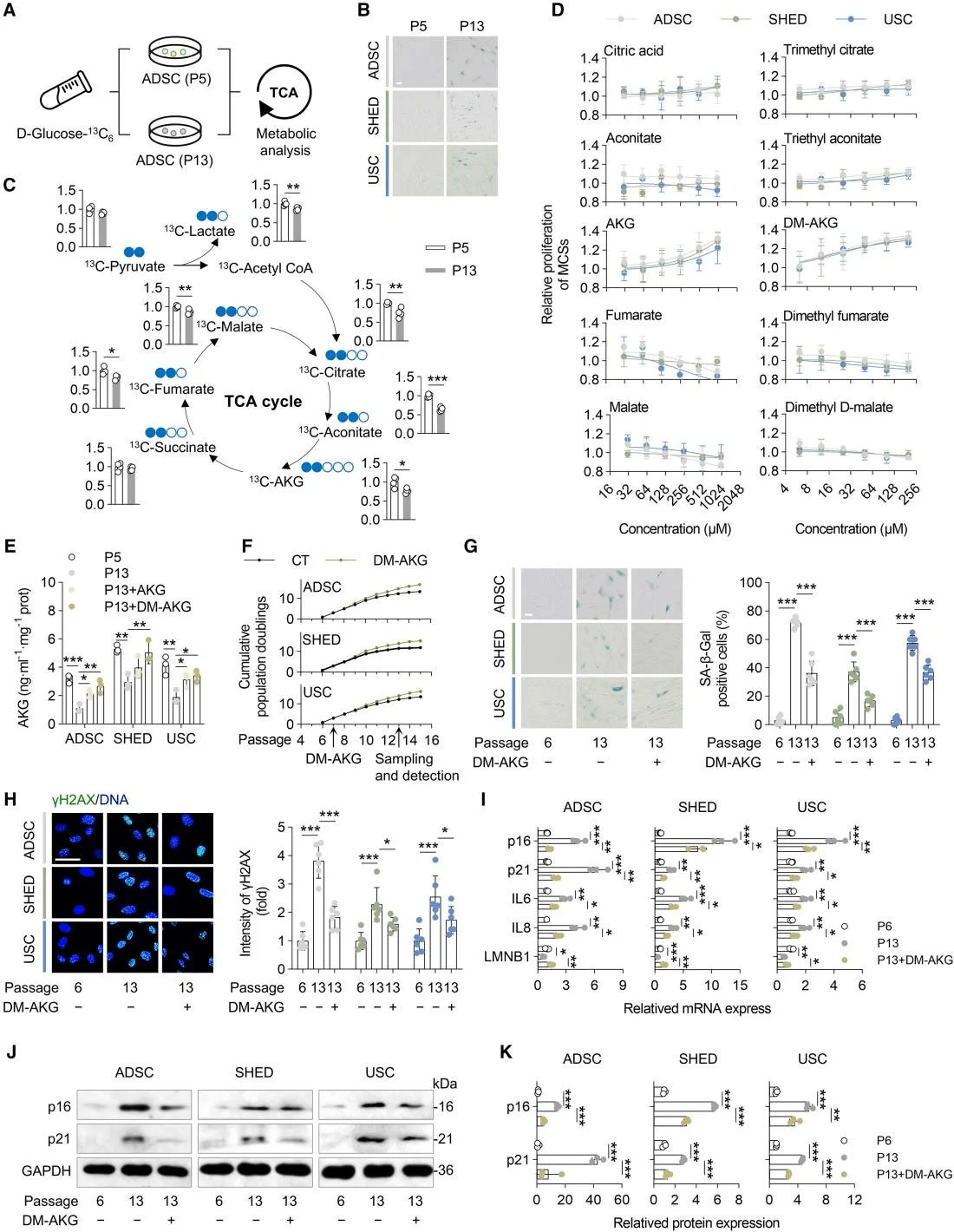
AKG identified as a key metabolite in mitigating mesenchymal stem cell (MSC) aging.
To elucidate the mechanism by which AKG delays MSC senescence, the researchers employed a thermal proteome profiling–cellular thermal shift assay (TPP–CETSA) to identify AKG’s protein targets. The results revealed that AKG predominantly targets proteins associated with ribosomal function, with ribosomal protein S23 (RPS23) being the most significantly affected. Given that RPS23 undergoes hydroxylation, the team investigated whether AKG influences this modification and found that AKG markedly enhanced RPS23 hydroxylation.Previous studies have established that OGFOD1 catalyzes proline hydroxylation of RPS23 in eukaryotes, with AKG serving as an essential cofactor. Further analyses confirmed that OGFOD1, rather than RPS23, directly binds AKG, and functional assays in MSCs validated OGFOD1’s critical role in this process. Collectively, these findings indicate that AKG delays MSC senescence by promoting RPS23 hydroxylation, with OGFOD1 acting as the key target mediating this modification.
Using co-immunoprecipitation (Co-IP) and bimolecular fluorescence complementation (BiFC) assays, the researchers found that DM-AKG strengthened the interaction between RPS23 and OGFOD1. They further constructed a molecular docking model of the RPS23–OGFOD1–AKG complex and validated the findings from molecular dynamics simulations using MSCs expressing the RPS23-P62A mutant. These experiments revealed that AKG suppresses MSC senescence by stabilizing the OGFOD1–RPS23 complex, with the hydroxylation state of Pro62 serving as a critical regulatory node in this process.
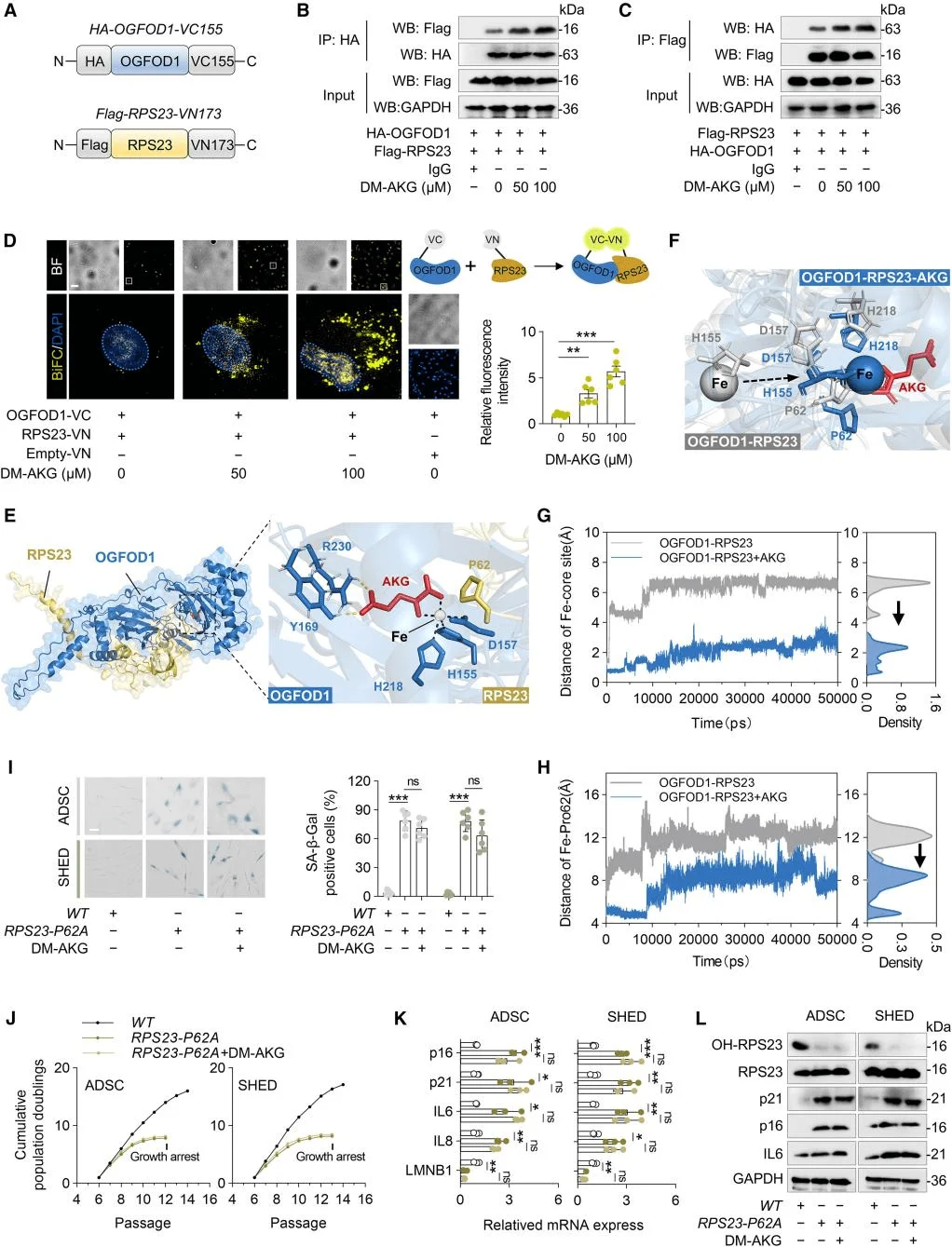
AKG promotes the formation of the OGFOD1–RPS23 complex, thereby enhancing the hydroxylation of RPS23 at Pro62.
Given that AKG significantly enhances MSC proliferation, the researchers investigated the proteins within the TCA cycle responsible for AKG production. With increasing MSC passage number, the expression of senescence markers p16 and p21 was markedly elevated. Among the TCA cycle enzymes involved in AKG synthesis, only IDH1 exhibited a significant decline in expression. The researchers then examined the impact of IDH1 expression on AKG production and MSC senescence, while also assessing the roles of IDH1 isoenzymes IDH2 and IDH3. The results confirmed that IDH1 acts as a protective factor against MSC senescence and indicated that reduced AKG levels caused by decreased IDH1 expression are a key driver of MSC aging.
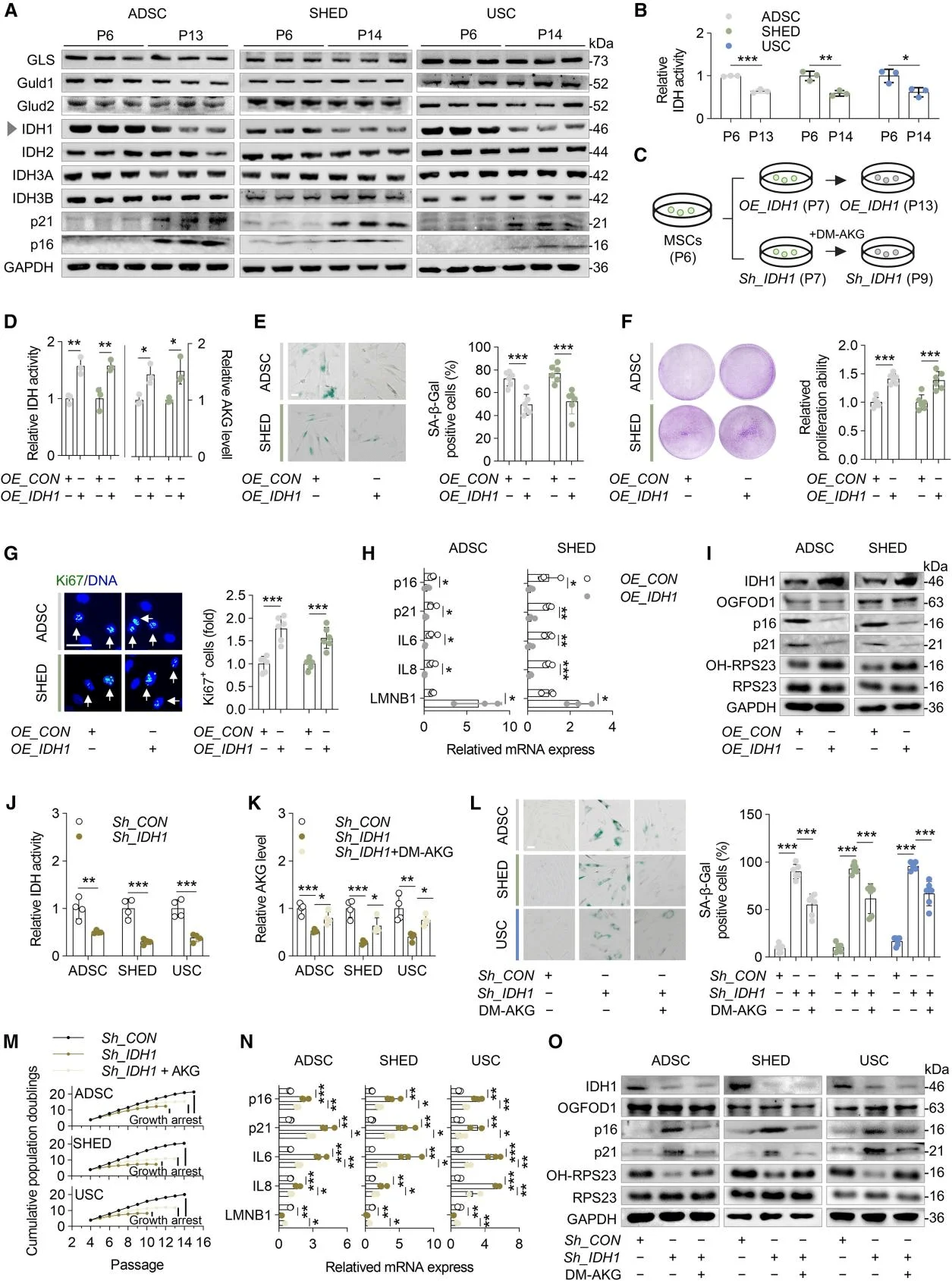
IDH1 regulates AKG synthesis, thereby modulating MSC senescence.
Accelerated cell proliferation generally depends on increased rates of protein synthesis. Stem cells, in particular, rely heavily on high levels of protein synthesis to maintain self-renewal and differentiation potential. The study found that with increasing passage number, protein synthesis rates in adipose-derived stem cells (ADSCs) significantly declined, whereas AKG markedly enhanced these rates.Furthermore, a decline in translational fidelity, not merely a reduction in protein synthesis speed, plays a critical role in aging. Senescent MSCs exhibited a significant accumulation of protein aggregates, which was effectively alleviated by AKG. Interestingly, while AKG could reverse the protein aggregation caused by IDH1 knockdown, it had no effect on aggregates induced by OGFOD1 knockdown or the RPS23-Pro62 mutation.
To probe this further, the researchers designed a dual-luciferase reporter system to monitor common translational errors and stop codon readthrough in MSCs, validating the system using, paromomycin a compound known to induce translational errors. The results demonstrated that blasticidin-induced disruption of proteostasis and MSC aging was independent of histone methylation and could not be reversed by high concentrations of AKG. These findings indicate that proteostasis imbalance represents an independent driver of MSC aging, and the regulation of protein homeostasis is a key mechanism through which AKG delays MSC senescence.
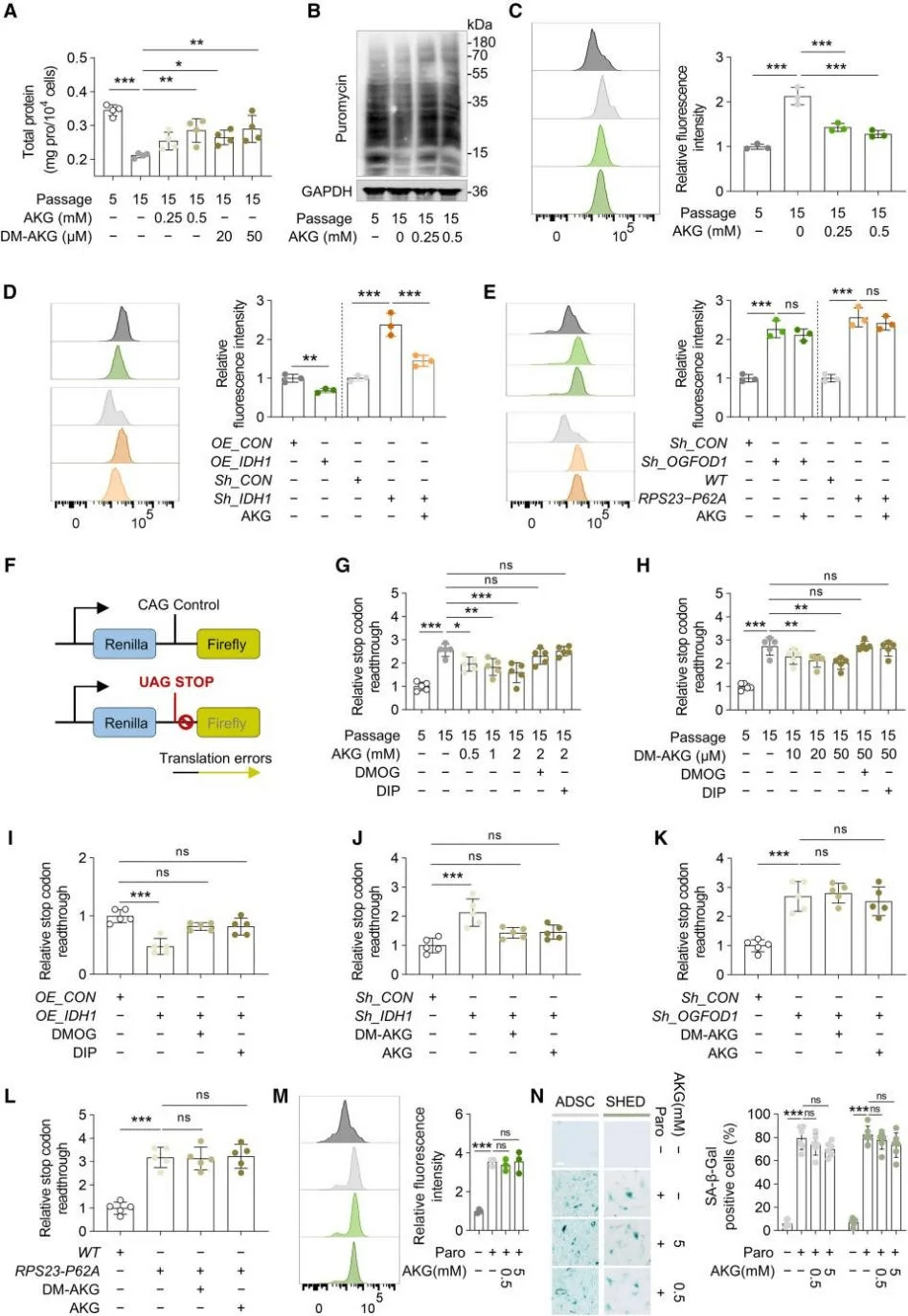
AKG enhances translational fidelity in MSCs by promoting OGFOD1-mediated hydroxylation of RPS23
Since IDH1 activation promotes AKG production, the researchers next focused on anti-aging strategies centered on IDH1 activation. They employed the naturally occurring IDH1 agonist, Scutellarin (Scu), which enhances IDH enzymatic activity in ADSCs and restores AKG levels in senescent MSCs. Scu exerts its effects by activating the IDH1-AKG-RPS23 signaling axis, thereby promoting MSC proliferation and delaying cellular senescence.To mimic the in vivo metabolic environment, the team developed a gut-liver organoid microfluidic system, demonstrating that Scu retains its anti-senescence efficacy after intestinal absorption and hepatic metabolism. In vivo experiments in aged mice further confirmed that Scu increases AKG levels, enhances RPS23 hydroxylation, and significantly ameliorates aging-associated phenotypes. These findings highlight the potential application of small-molecule IDH1 agonists in intervening in aging and age-related diseases.
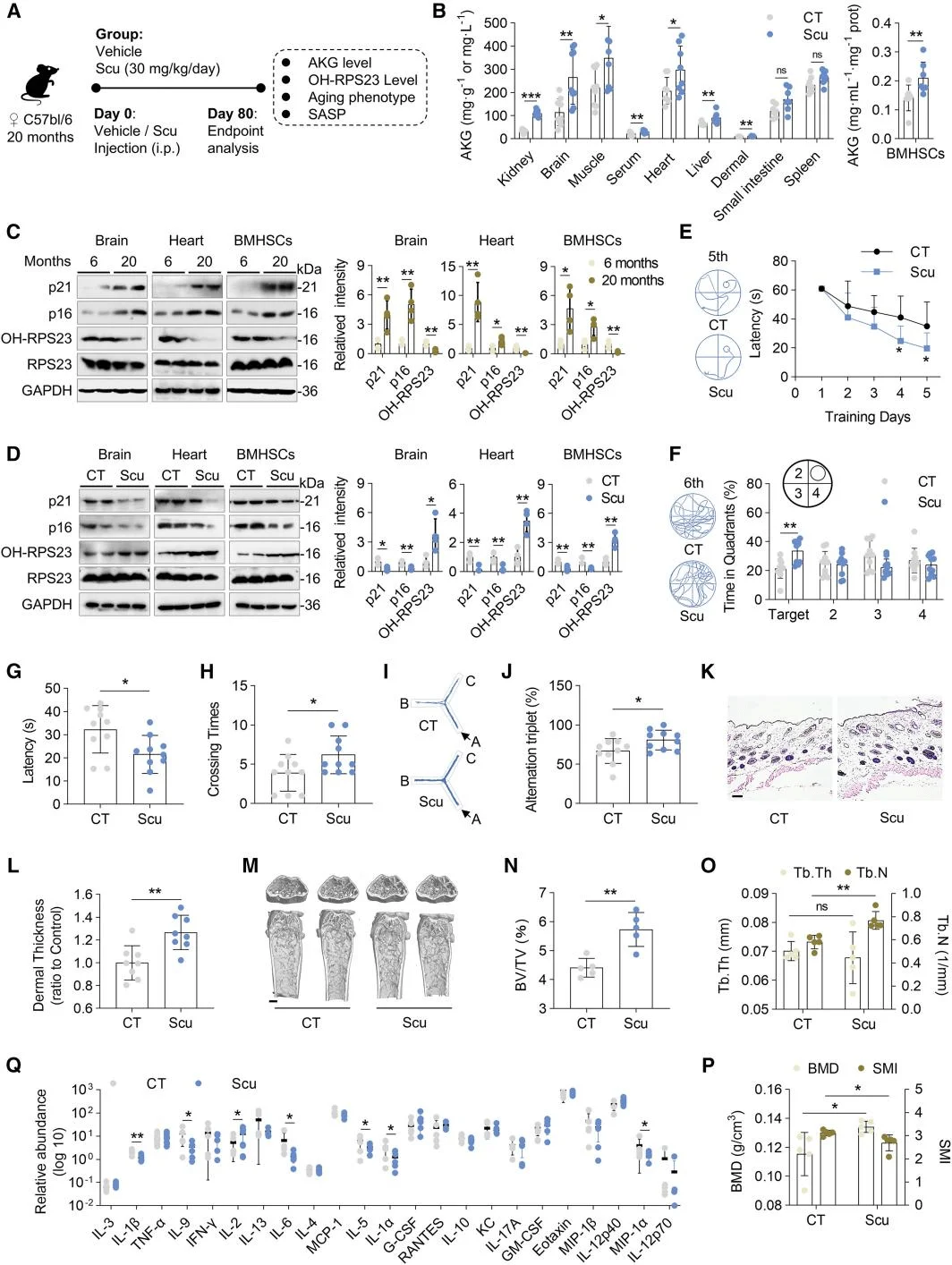
Scutellarin (Scu), an IDH1 agonist, ameliorates aging-associated phenotypes in aged mice.
In summary, this study reveals that AKG targets RPS23 to maintain protein homeostasis, thereby promoting MSC self-renewal and delaying cellular senescence. These findings integrate two critical hallmarks of aging—metabolic dysregulation and loss of protein homeostasis—providing a foundation for the development of potential anti-aging therapeutic strategies.
Support Provided by Ubigene
Ubigene provided essential support for this study. Utilizing human induced pluripotent stem cells (iPSCs) models supplied by Ubigene, the research team was able to integrate two core mechanisms of aging—metabolic dysregulation and loss of protein homeostasis—thereby uncovering their synergistic role in regulating cellular senescence. Ubigene’s critical contribution not only advanced the understanding of aging mechanisms but also laid a solid foundation for the development of potential anti-aging therapeutic strategies.
Reference
Cui Z, Li J, Li C, et al. Identifying the target, mechanism, and agonist of α-ketoglutaric acid in delaying mesenchymal stem cell senescence[J]. Cell Reports, 2025, 44(7).


