Ubigene Supports Breakthrough in Understanding the Link Between Dietary Fat and Cancer——Olive Oil-Based High-Fat Diet


Ubigene Supports Breakthrough in Understanding the Link Between Dietary Fat and Cancer——Olive Oil-Based High-Fat Diet-Induced Obesity Promotes TNBC Metastasis Through a Specific Molecular Pathway
According to data from the World Health Organization (WHO), breast cancer has become the most common cancer among women worldwide. Triple-negative breast cancer (TNBC), although accounting for only about 15% of invasive breast cancer cases, is responsible for approximately 40% of breast cancer–related deaths, making it the most aggressive subtype. Mounting evidence has demonstrated a strong association between obesity and poor prognosis in TNBC. Obese women have a 35–50% higher risk of developing TNBC compared with women of normal weight, and their risk of metastasis and mortality increases by 30–40%. However, the molecular mechanisms linking obesity and TNBC metastasis have remained unclear.
On October 7, a research team led by Anthony Avellino and Bing Li at the University of Iowa published a landmark study in Cancer Research entitled:“An Olive Oil-Based High-Fat Diet Promotes Obesity-Driven Metastasis of Triple-Negative Breast Cancer.”This study aimed to elucidate how different types of high-fat diets (HFD) drive obesity and influence TNBC progression and the underlying molecular pathways. Notably, the Fabp5 knockout cell line (4T1) and FABP5 knockout cell line (MDA-MB-231) used in the study were provided by Ubigene, enabling the precise dissection of FABP5’s role in oleic acid (OA)-mediated PKC/ALDH pathway activation and TNBC metastasis.
Using mouse models, in vitro cell experiments, and human data analyses, the researchers demonstrated that cocoa butter–based HFD and olive oil–based HFD induced comparable levels of obesity in mice; however, only the olive oil–based HFD enhanced TNBC stem-like properties and lung metastasis. Mechanistically, oleic acid (OA), a major component of olive oil, activated the PKC–ALDH signaling pathway, thereby promoting TNBC metastasis. Crucially, fatty acid–binding protein 5 (FABP5) in TNBC cells was identified as a key mediator of OA-induced pathway activation. In multiple mouse models, loss of FABP5 markedly reduced TNBC metastasis, while clinical data analysis showed FABP5 overexpression was associated with poor prognosis in TNBC patients.
Taken together, this study provides compelling evidence that an olive oil–based high-fat diet promotes obesity-associated TNBC metastasis via the OA/FABP5-driven oncogenic signaling axis, offering a strong scientific rationale for precision dietary intervention and targeted therapeutic strategies in TNBC.

Research Highlights
Triple-negative breast cancer (TNBC) is a highly aggressive breast cancer subtype with relatively poor prognosis. Its name derives from the absence of three key receptor proteins on tumor cells: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). TNBC is more common in younger women (particularly those under 40), women of African descent, and individuals carrying BRCA1 mutations. Compared with women of normal weight, obese women—especially postmenopausal—have a significantly higher risk of developing TNBC.
Obesity is often attributed to excessive caloric intake, particularly from high-fat diets (HFDs). According to the U.S. National Health and Nutrition Examination Survey (NHANES), the average daily caloric intake of Americans is approximately 2100 kcal, with 40% derived from fat, primarily saturated and monounsaturated fatty acids. Because human diets are compositionally complex, it remains unclear whether saturated and unsaturated fats contribute equally to obesity prevalence, and whether obesity induced by different types of dietary fat differentially affects the progression of obesity-associated diseases, including TNBC.
A hallmark of obesity is excess adipose tissue accumulation and elevated circulating lipids. Due to their hydrophobicity, long-chain dietary fatty acids require fatty acid–binding proteins (FABPs) for solubilization, transport, and metabolism. The FABP family comprises at least nine members, each displaying tissue-specific expression patterns—e.g., FABP2 in the intestine, FABP4 in adipose tissue, and FABP5 in epithelial tissues. Previous studies have linked FABP4 to obesity-related disorders including cardiovascular disease, diabetes, and breast cancer. FABP5, which is highly expressed in epithelial cells and epithelial-derived tumors, mediates fatty acid uptake and oxidation and activates oncogenic signaling pathways (e.g., PPARβ/δ), thereby promoting tumor progression and invasion. Importantly, FABPs undergo allosteric conformational changes upon binding fatty acids of different saturation, leading to the activation of unique lipid-mediated signaling pathways. This study was therefore designed to investigate how different types of high-fat diets (HFD) induce obesity and influence TNBC progression and its molecular mechanisms.
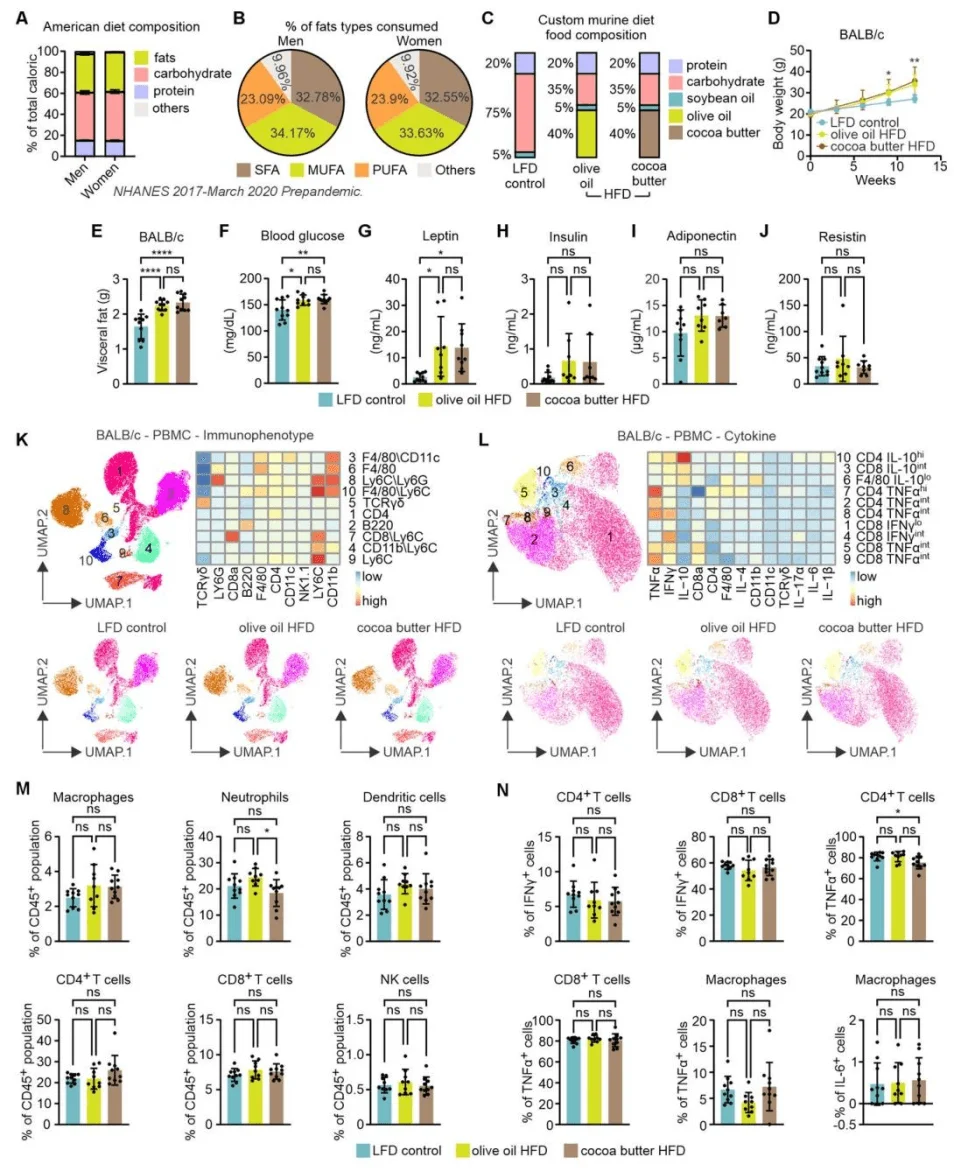
Cocoa butter– and olive oil–based high-fat diets induce comparable levels of obesity
The research team first examined whether obesity induced by different types of dietary fat has distinct effects on TNBC progression. Two HFD formulations were used, each providing 40% of calories from fat—either saturated fat–rich cocoa butter or monounsaturated fat–rich olive oil—with a low-fat diet (LFD) serving as control. The impact of these diets on obesity was evaluated using BALB/c and C57BL/6 mouse models. Results showed that after 12 weeks, both HFD groups exhibited significantly increased body weight and visceral fat compared with the LFD group in BALB/c mice. Serum glucose and leptin levels were elevated, insulin levels showed an increasing trend, while adiponectin and resistin levels remained unchanged. No significant changes were observed in the frequencies of major immune cell subsets in peripheral blood mononuclear cells (PBMCs) or in their cytokine production profiles.
Similar results were obtained in C57BL/6 mice. Both HFD groups displayed comparable body weight gain; NMR body composition analysis revealed increased fat mass and decreased lean mass. Gene expression in adipose depots indicated downregulation of both lipogenesis- and lipolysis-related genes, and no major alterations were detected in peripheral immune cell populations or their cytokine production. Collectively, these findings indicate that HFDs rich in either saturated (cocoa butter) or monounsaturated (olive oil) fatty acids induce similar degrees of obesity in mice, with minimal impact on circulating immune cell phenotypes and functions.
Olive oil–based high-fat diet–induced obesity promotes TNBC lung metastasis, whereas cocoa butter–based high-fat diet does not
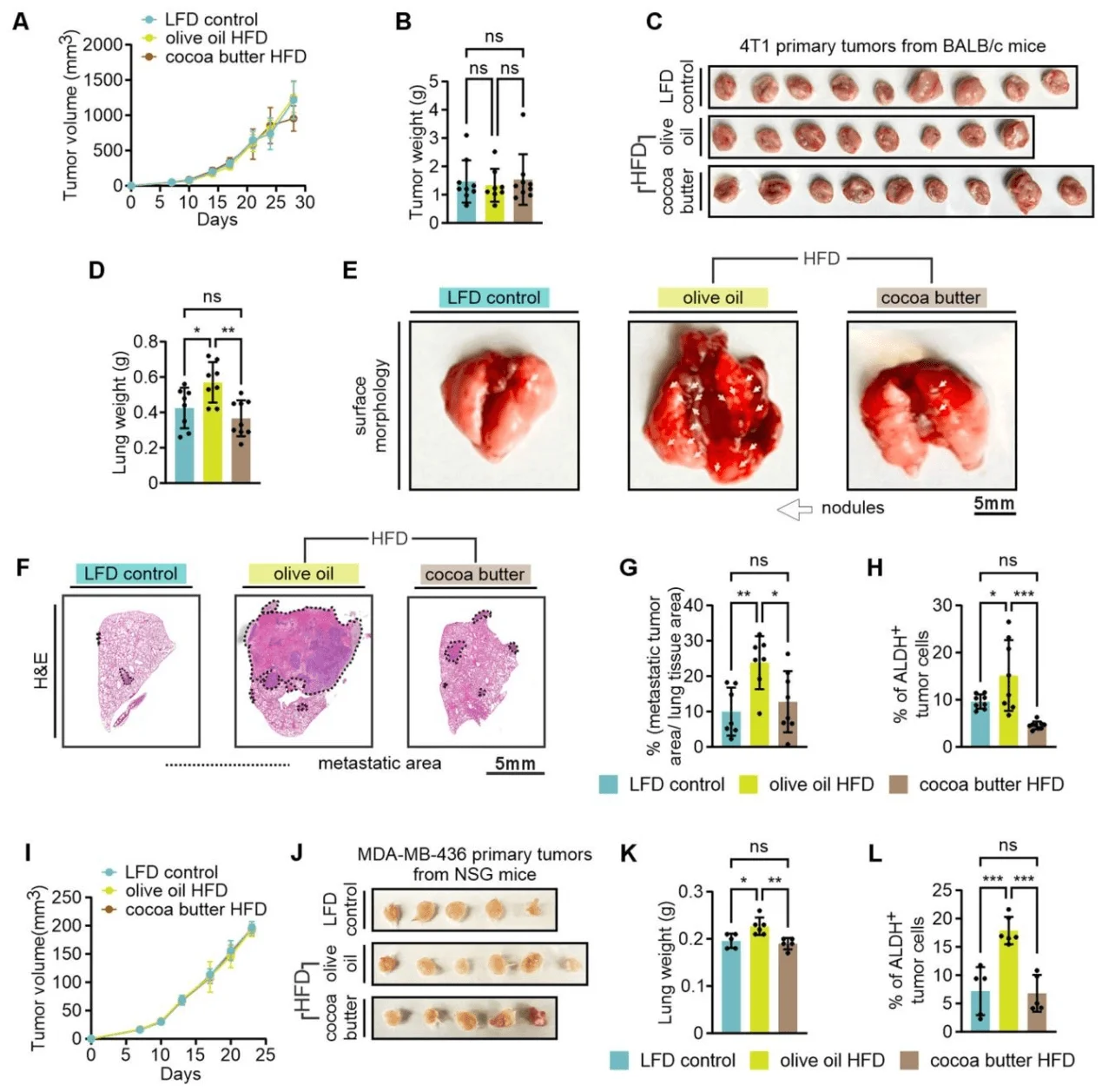
The researchers next evaluated whether different high-fat diets (HFDs) differentially affect TNBC metastasis. 4T1 TNBC cells were orthotopically implanted into immunocompetent BALB/c mice, while human TNBC cell lines (MDA-MB-436 and MDA-MB-231) were implanted into immunodeficient NSG mice. Mice were fed one of three diets—low-fat diet (LFD), cocoa butter–based HFD, or olive oil–based HFD—and tumor growth and lung metastasis were monitored.
In the 4T1 TNBC model, no significant differences were observed among the three diet groups in primary tumor growth curves, tumor weights, or tumor sizes. However, mice fed the olive oil–based HFD exhibited increased lung weights, greater numbers of visible metastatic nodules on lung surfaces, and more extensive metastatic lesions confirmed by histological staining. Furthermore, aldehyde dehydrogenase (ALDH) activity, a recognized marker of breast cancer stemness and metastasis, was significantly elevated in tumors from the olive oil–HFD group.
Consistent with these findings, in the MDA-MB-436 xenograft model, the olive oil–based HFD did not alter primary tumor growth or final tumor size, but markedly enhanced lung metastasis and increased tumor ALDH activity. Similarly, in the MDA-MB-231 model, primary tumor growth and size were comparable among groups; however, the olive oil–HFD group displayed a pronounced increase in lung metastasis. Remarkably, the number of circulating tumor cells (CTCs) was already significantly higher in this group as early as day 1 after orthotopic implantation into the mammary fat pad, although pulmonary vascular permeability remained unchanged.
Immune profiling analysis revealed no significant differences among the diet groups in the proportions of immune cell subsets or cytokine production within the tumor stroma.Collectively, these findings indicate that olive oil–based HFD–induced obesity promotes TNBC progression primarily by enhancing lung metastasis rather than stimulating primary tumor growth. This pro-metastatic effect appears to arise through direct modulation of tumor cell behavior—increasing early metastatic seeding and invasive capacity—rather than via immune-mediated mechanisms.
Elevated serum oleic acid (OA) in mice fed an olive oil–based high-fat diet enhances TNBC invasiveness
Given the high oleic acid (OA) content of olive oil, the research team investigated whether olive oil–based high-fat diet (HFD)-induced obesity promotes TNBC metastasis through elevated serum OA levels. Metabolomic profiling of serum from mice fed olive oil–HFD or low-fat diet (LFD) was performed, and the effect of these sera on TNBC cell invasion was assessed using Transwell invasion assays.
Gas chromatography–mass spectrometry (GC–MS) analysis revealed that the serum metabolomic profile of the olive oil–HFD group was distinct, characterized by a significant increase in unsaturated fatty acids, particularly oleic acid, linoleic acid, and arachidonic acid. Among these, oleic acid was the most significantly elevated metabolite compared with both the LFD group and the cocoa butter–HFD group. OA levels were positively correlated with other upregulated metabolites including linoleic acid, glycerol, arachidonic acid, and inositol, implicating pathways related to nucleotide biosynthesis, inositol phosphate metabolism, and unsaturated lipid biosynthesis. In contrast, levels of saturated fatty acids, amino acids, and metabolites associated with glycolysis and the TCA cycle showed no significant changes.
Functionally, Transwell invasion assays demonstrated that serum from olive oil–HFD–fed mice significantly enhanced the invasive capacity of both 4T1 and MDA-MB-231 TNBC cells. By contrast, serum from cocoa butter–HFD or LFD–fed mice had no significant effect on invasion.These findings indicate that olive oil–based HFD elevates serum oleic acid levels, and this increase in OA plays a key role in promoting TNBC metastatic potential.

Oleic acid directly promotes TNBC cell invasion in vitro
To further evaluate the direct effect of oleic acid (OA) on the invasiveness of triple-negative breast cancer (TNBC) cells, the researchers conducted a series of in vitro assays. Differentiated 3T3 adipocytes were treated with bovine serum albumin (BSA)-conjugated OA, palmitic acid (PA), or BSA alone, and lipid droplet formation was visualized by Oil Red O staining. Additionally, a co-culture system was established in which TNBC cells were seeded in collagen-coated inserts (upper chamber), while differentiated adipocytes were placed in the lower chamber. In parallel, TNBC cells were treated directly with OA, PA, or BSA to assess their invasive potential.
The results showed that both OA and PA induced significant lipid droplet accumulation in 3T3 adipocytes. However, adipocytes differentiated in the presence of OA most strongly enhanced the invasion of both 4T1 and MDA-MB-231 TNBC cells, compared with adipocytes differentiated with PA or BSA.Furthermore, direct OA treatment markedly increased the invasiveness of 4T1 and MDA-MB-231 cells, while PA treatment had no such effect. OA exposure also promoted lipid accumulation and proliferation in TNBC cells, and upregulated the expression of key invasion- and stemness-associated markers, including Bmi-1, Zeb2, Mmp9, and Vimentin.
These findings demonstrate that oleic acid can directly enhance TNBC cell invasiveness in vitro, mirroring the pro-metastatic effects observed in vivo under the olive oil–based high-fat diet condition.

Oleic acid drives TNBC cell invasion through activation of the PKC–ALDH signaling pathway
Previous in vivo results demonstrated that mice fed an olive oil–based high-fat diet (HFD) exhibited increased aldehyde dehydrogenase (ALDH) activity and enhanced lung metastasis of triple-negative breast cancer (TNBC). These findings prompted the research team to investigate whether oleic acid (OA) directly promotes TNBC cell invasion by activating ALDH-related signaling.
To test this hypothesis, 4T1 and MDA-MB-231 TNBC cells were treated with varying concentrations of OA or other dietary fatty acids, including palmitic acid (PA), linoleic acid, and arachidonic acid. ALDH activity was measured following treatment. Additionally, a series of pathway-specific inhibitors were applied—such as DEAB (an ALDH inhibitor), S3I-201 (a STAT3 inhibitor), Etomoxir (a fatty acid oxidation inhibitor), and sotrastaurin (a PKC inhibitor)—to dissect the molecular mechanism underlying OA-induced TNBC invasion. The expression of stemness-related genes and PKC-associated genes, as well as PKC protein levels, was also analyzed.The results revealed that OA enhanced ALDH activity in a dose-dependent manner in 4T1 cells, and that OA-induced ALDH activity was higher than that induced by other fatty acids in both 4T1 and MDA-MB-231 cells. Inhibition of ALDH activity by DEAB markedly reduced OA-induced cell invasion, confirming the role of ALDH in mediating OA’s pro-invasive effects.
Among the signaling inhibitors tested, only sotrastaurin, the PKC inhibitor, significantly suppressed OA-induced ALDH activation in 4T1 cells. OA treatment also increased the expression of stemness-associated genes (such as Aldh2 and Vimentin) and PKC-related genes (including PLCγ1 and PKCε). Western blotting further confirmed that OA specifically enhanced PKCε protein expression. Importantly, sotrastaurin inhibited OA-induced ALDH activity in a dose-dependent manner without affecting cell viability, while also significantly reducing OA-induced TNBC cell invasion and downregulating invasion-related markers, such as Vimentin.
Collectively, these findings demonstrate that oleic acid promotes TNBC cell invasion by activating the PKC–ALDH signaling axis, with PKC activation serving as a critical upstream regulator of the OA/ALDH pathway.

FABP5 mediates oleic acid–induced activation of the PKC–ALDH signaling pathway in TNBC cells
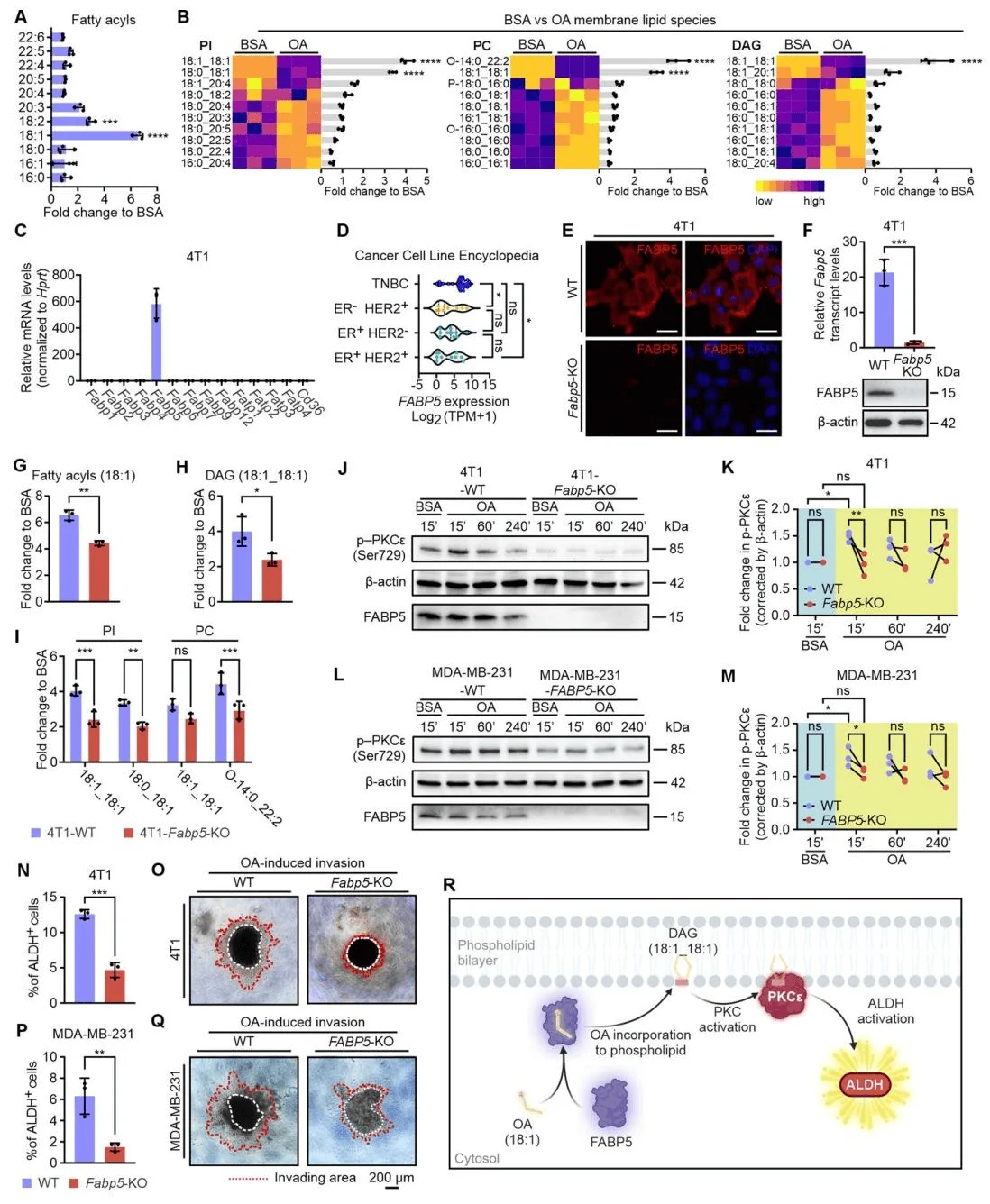
To elucidate how oleic acid (OA) activates protein kinase C (PKC) in triple-negative breast cancer (TNBC), researchers treated 4T1 cells with either OA or bovine serum albumin (BSA) alone and performed untargeted lipidomic profiling using liquid chromatography–mass spectrometry (LC–MS). The analysis revealed that OA treatment markedly increased the overall levels of 18:1 acyl chains in 4T1 cells, which were incorporated into multiple membrane lipid classes—such as phosphatidylinositol (PI), phosphatidylcholine (PC), and diacylglycerol (DAG)—at both the sn-1 and sn-2 positions. Fatty acid–binding protein 5 (FABP5) was identified as the predominant FABP isoform expressed in both 4T1 and MDA-MB-231 TNBC cells. Consistent with this, the CCLE (Cancer Cell Line Encyclopedia) database confirmed high FABP5 expression across TNBC cell lines, while CD36 and other fatty acid transport proteins (FATPs) were not upregulated.
Functional studies using FABP5 knockout cell lines—generated by Ubigene (Guangzhou, China)—demonstrated that FABP5 loss substantially reduced OA-mediated incorporation of 18:1 acyl chains and DAG formation, resulting in decreased levels of membrane lipids such as PI and PC. Western blotting showed that FABP5 knockout significantly attenuated OA-induced PKCε activation in both 4T1 and MDA-MB-231 cells. Furthermore, ALDH activity assays and Matrigel invasion experiments revealed that FABP5 deficiency greatly diminished OA-induced ALDH activity and invasive potential of TNBC cells.Collectively, these findings establish FABP5 as a critical mediator of OA-induced PKC–ALDH activation in TNBC cells. By facilitating the incorporation of OA-derived acyl chains into membrane lipids, FABP5 promotes PKC activation and downstream ALDH signaling, thereby driving TNBC cell invasion and metastatic potential.
FABP5 promotes TNBC progression in mouse models and correlates with poor prognosis in human patients
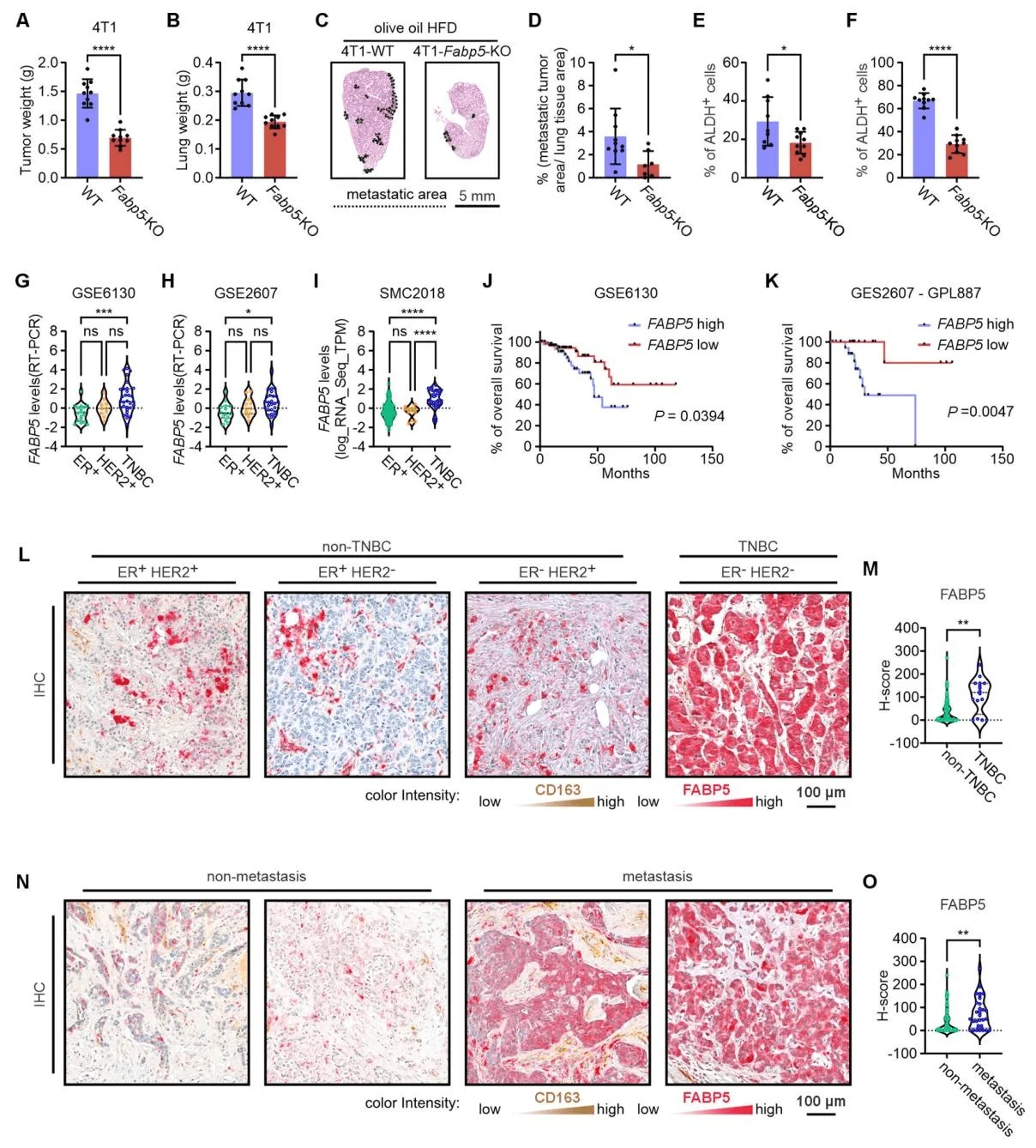
To further clarify the in vivo role of fatty acid–binding protein 5 (FABP5) in promoting triple-negative breast cancer (TNBC) progression, researchers orthotopically injected wild-type (WT) and FABP5 knockout (KO) 4T1 tumor cells into the mammary fat pads of BALB/c mice fed an olive oil–based high-fat diet (HFD), and monitored both primary tumor growth and lung metastasis. In the 4T1 model, FABP5 knockout significantly inhibited primary tumor growth, reduced lung weight and metastatic burden, and lowered ALDH activity in both primary and metastatic tumors. Consistently, in the MDA-MB-231 model, FABP5 deficiency markedly decreased primary tumor growth, lung weight, and metastasis, reduced tumor ALDH activity, and did not affect proliferation, but significantly impaired invasive capacity.
The team further analyzed FABP5 expression and its correlation with overall survival across different breast cancer subtypes in publicly available human datasets and in a cohort of 96 surgically resected breast cancer samples. Across multiple datasets (GSE6130, GSE2607, SMC2018), FABP5 expression was significantly higher in TNBC compared with ER+ or HER2+ breast cancers. Kaplan–Meier survival analysis showed that high FABP5 expression was associated with shorter overall survival, both in datasets comprising multiple breast cancer subtypes and in TNBC-specific cohorts.
Analysis of human tissue samples confirmed that FABP5 is predominantly localized in tumor cells, rather than in CD163+ macrophages. TNBC tumors exhibited significantly higher FABP5 expression than non-TNBC subtypes, and high FABP5 expression correlated positively with metastasis, whereas FABP4 showed no such association.Together, these findings establish FABP5 as a key driver of TNBC progression, particularly in promoting metastatic dissemination, highlighting its potential as a prognostic biomarker and a therapeutic target in TNBC.
Support Provided by Ubigene
The FABP5 knockout cell lines used in this study—4T1 Fabp5 KO and MDA-MB-231 FABP5 KO—were provided by Ubigene. Both cell lines were validated at the protein level by Western blotting, providing essential tools for elucidating FABP5 as a prognostic biomarker and potential therapeutic target in TNBC.
Ubigene currently provides over 8,000 gene knockout cell lines, with prices starting at $990 and ready-to-ship cells available within one week. Researchers can explore the Ubigene KO Cell Line Bank to locate the cell lines they need. For targets not available in the cell line bank, Ubigene offers custom gene knockout services. Utilizing its proprietary CRISPR-U™ technology, Ubigene can increase gene-editing efficiency by 10–20×, delivering highly efficient and reliable tools for functional genomics research to institutions worldwide.
Contact us for more technical support >>>
Reference
Avellino A, Jiang X, Lee M, Yu J, Liu S, Han X, Li J, Shilyansky J, Wang Z, Curry M, Xiong Y, Lizarraga IM, Huang Y, Sugg SL, Hao J, Li B. An Olive Oil-Based High-Fat Diet Promotes Obesity-Driven Metastasis of Triple-Negative Breast Cancer. Cancer Res. 2025 Sep 5:10.1158/0008-5472.CAN-25-0822. doi: 10.1158/0008-5472.CAN-25-0822. Epub ahead of print. PMID: 40911782; PMCID: PMC12502211.



