IF = 14.1 | Ubigene Supports Research Showing CRC Cell–Derived Exosomal PIK3CA Mutations Activate Fibroblasts and Enhance Tumor Metastasis


In May 2025, the team led by Prof. Fang Jin at China Medical University published a study in Advanced Science titled Colorectal Cancer Cells-Derived Exosomal PIK3CA Mutation DNA Promotes Tumor Metastasis by Activating Fibroblast and Affecting Tumor Metastatic Microenvironment.” With comprehensive technical support from Ubigene, the researchers performed precise gene editing on LS174T cells carrying the PIK3CA H1047R mutation by converting the nucleotide at position 3140 from G to A, resulting in the substitution of Arg1047 with His. This enabled the generation of a PIK3CA wild-type (WT) cell line, which was then rigorously compared with the original mutation-type (MT) cells.This collaborative work contributed to uncovering the mechanism by which colorectal cancer cell-derived exosomes deliver PIK3CA mutations to fibroblasts, thereby activating fibroblasts, altering the metastatic tumor microenvironment, and ultimately promoting tumor metastasis.

Research has demonstrated that PIK3CA-mutant DNA contained in colorectal cancer (CRC)-derived exosomes can be transferred into recipient fibroblasts, where it undergoes transcription and translation. The resulting protein interacts with the endogenous p85 regulatory subunit of the phosphoinositide 3-kinase (PI3K) pathway, ultimately activating fibroblasts and converting them into cancer-associated fibroblasts (CAFs). These CAFs promote tumor cell metastasis both in vitro and in vivo and secrete high levels of IL-6.Moreover, PIK3CA mutations have been detected in CAFs at both primary and metastatic tumor sites, indicating that the mutation may facilitate tumor metastasis by reshaping the tumor microenvironment (TME). Consistently, CRC patients harboring PIK3CA mutations exhibit elevated serum IL-6 levels and show a higher propensity for metastatic progression.
Collectively, these findings suggest that the combined detection of serum exosomal PIK3CA mutations and serum IL-6 may serve as a promising diagnostic tool for CRC. Furthermore, co-targeting PIK3CA mutations and IL-6 may represent a novel therapeutic strategy for managing CRC.
Intercellular communication is a critical driver of tumor progression and metastasis. Tumor cell-derived exosomes can transport tumor-specific biomolecules to stromal cells, thereby disrupting the homeostasis of the tumor microenvironment (TME) and promoting cancer progression and dissemination. Extensive studies have shown that, during tumor evolution, cancer cells can recruit fibroblasts and induce their conversion into cancer-associated fibroblasts (CAFs) . CAFs remodel the TME by secreting various cytokines and extracellular matrix components, consequently regulating multiple aspects of tumor development—including enhancing tumor cell proliferation, influencing invasion and metastasis, and contributing to chemotherapy resistance.
Although tumor-derived exosomes are known to activate fibroblasts and participate in shaping the TME, most current research focuses on the roles of proteins and RNAs within exosomes. In contrast, significantly less attention has been given to how exosomal aberrant DNA influences TME homeostasis and contributes to tumor progression and metastatic spread. While the functional role of PIK3CA mutations within tumor cells has been largely elucidated, therapeutic strategies directly targeting PIK3CA mutations in cancer cells have shown limited efficacy in preventing colorectal cancer (CRC) metastasis.
This study aims to investigate whether PIK3CA-mutant DNA can be transferred from tumor cells to stromal cells via exosomes and whether this form of genetic communication further promotes metastatic progression in CRC.
Research Findings
1.Exosomes Derived from PIK3CA-WT and PIK3CA-MT Cells Contain PIK3CA Mutational Profiles Identical to Their Parental Cells
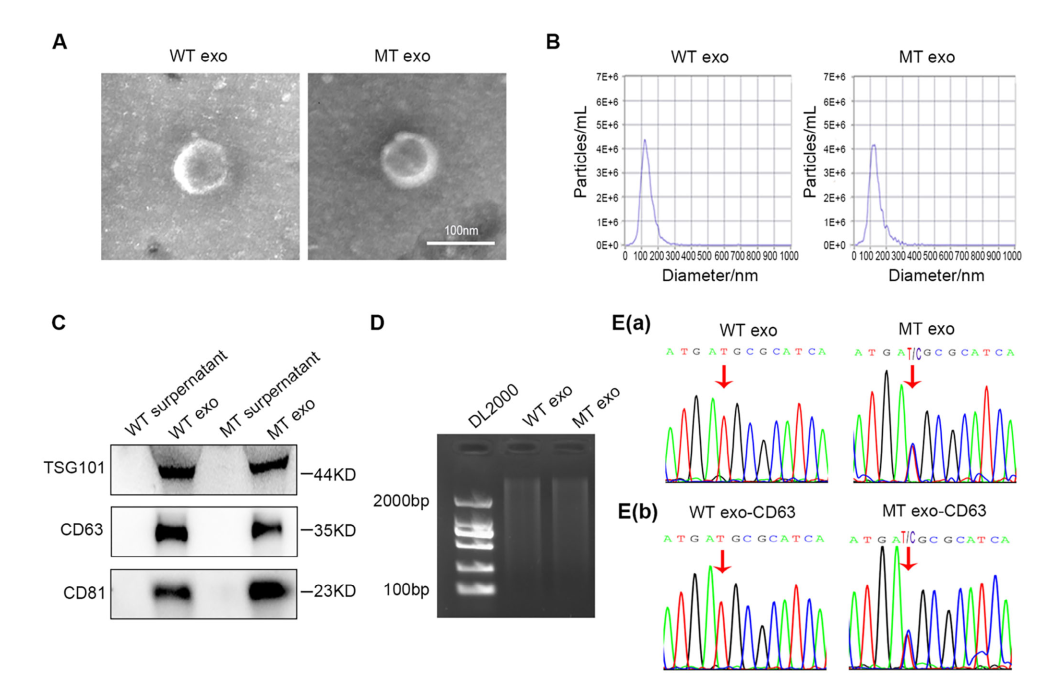
Figure 1. Exosomes Derived from WT and MT Cells Exhibit the Same PIK3CA Mutation Status as Their Parental Cells
The study first confirmed that exosomes derived from MT cells contain the PIK3CA mutation, whereas exosomes from WT cells do not, indicating that exosomes faithfully carry the same PIK3CA mutational status as their parent cells. To further ensure that the extracted DNA originated specifically from exosomes rather than from other types of extracellular vesicles, the research team incubated exosomes with magnetic beads coated with the exosome-specific marker CD63 antibody, followed by DNA extraction from the bead-captured exosomes. This approach ensured that the DNA analyzed was derived exclusively from CD63-positive exosomes.Subsequent analysis further verified that MT-cell–derived exosomes harbor the identical PIK3CA mutation present in the parental MT cells.
2. PIK3CA Mutations Carried by MT-Cell–Derived Exosomes Can Be Horizontally Transferred to Fibroblasts
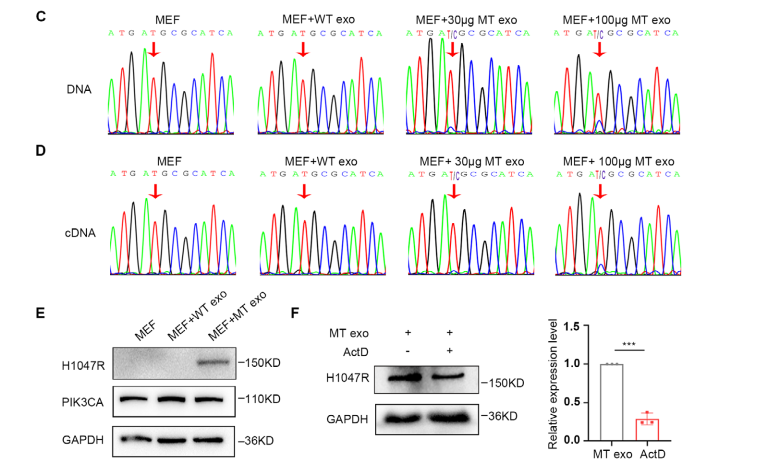
Figure 2. Horizontal Transfer of the PIK3CA H1047R Mutation from MT-Cell-Derived Exosomes to Fibroblasts
To further validate that the PIK3CA mutation contained in MT-cell–derived exosomes can be horizontally delivered to MEF cells, the research team co-incubated exosomes from both WT and MT cells with MEF cells. The results showed that PIK3CA-mutant DNA from MT-derived exosomes was successfully transferred into MEF cells, where it underwent transcription and translation. To exclude potential effects mediated by exosomal RNA or proteins, MEF cells were treated with the transcriptional inhibitor ActD and then co-incubated with MT-cell–derived exosomes. The findings revealed that approximately 75% of the mutant protein expression in MEF cells originated predominately from the horizontally transferred exosomal PIK3CA DNA, indicating that DNA-mediated transfer plays the major role.
3.Exosomal PIK3CA Mutations Activate MEFs and Induce Their Conversion into CAFs
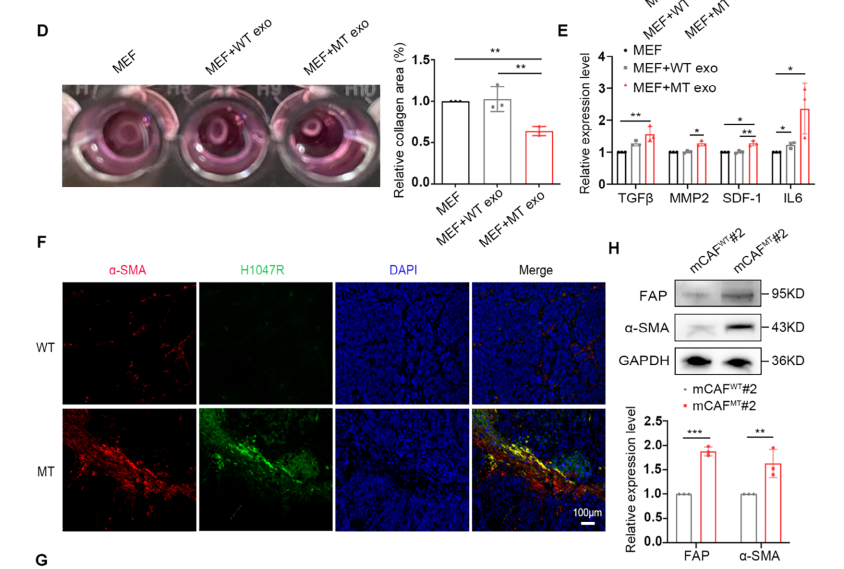
Figure 3. Exosomal PIK3CA H1047R Mutation Activates Mouse Embryonic Fibroblasts (MEFs) and Induces Their Conversion into Cancer-Associated Fibroblasts (CAFs)
Following treatment of MEF cells with MT-cell–derived exosomes, both the contractile capacity and cytokine secretion of MEFs were markedly enhanced. These findings indicate that the horizontal transfer of the PIK3CA mutation via MT-derived exosomes confers a strong potential to activate MEFs and drive their transition into CAFs. In vivo experiments further demonstrated co-localization of α-SMA and H1047R expression within CAFs, providing additional evidence that fibroblast activation is closely associated with the horizontal transfer of the PIK3CA mutation.
4.Exosomal PIK3CA Mutations Are Associated with CRC Metastasis
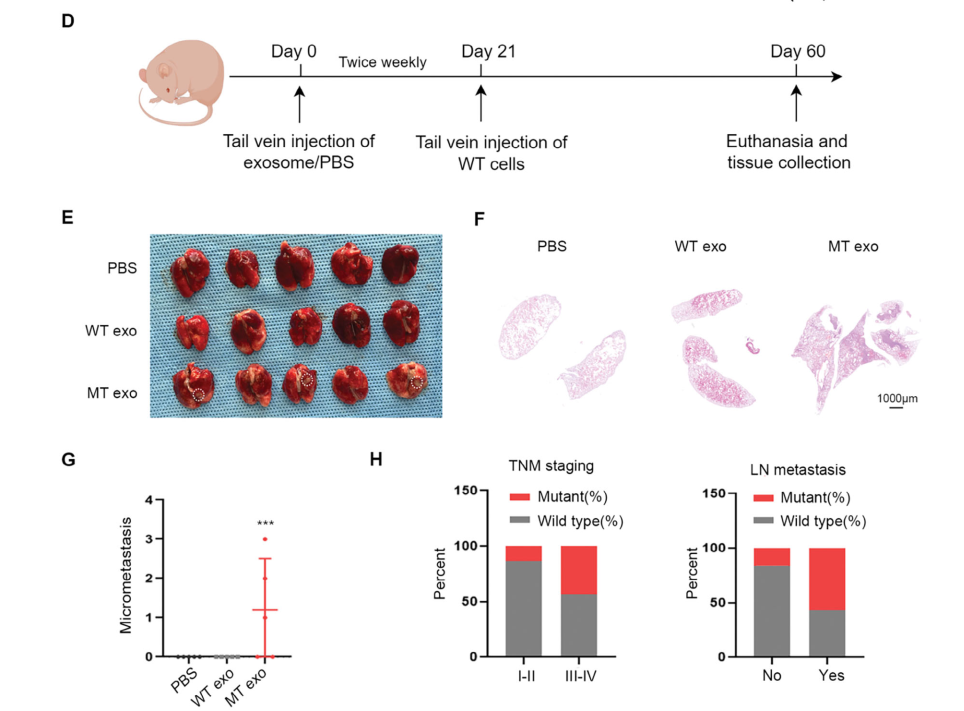
Figure 4. Exosomal PIK3CA H1047R Mutation Is Associated with Colorectal Cancer Metastasis
Exosomes can also establish a pre-metastatic niche in distant organs, reating conditions for tumor cells to disseminate from the primary site. To investigate the potential role of exosomal PIK3CA DNA in pre-metastatic niche formation, the research team injected WT and MT cells via the tail vein into nude mice. Metastatic foci were observed in the lung tissue of mice receiving MT-cell-derived exosomes, indicating that exosomal PIK3CA DNA can activate fibroblasts in distant organs, thereby promoting the spread of tumor cells from the primary site.Consistently, analysis of CRC patients suggested that exosomal PIK3CA DNA mutations are clinically associated with CRC metastasis.
5.Horizontal Transfer of Exosomal PIK3CA Mutations Induces CAFs to Secrete High Levels of IL-6
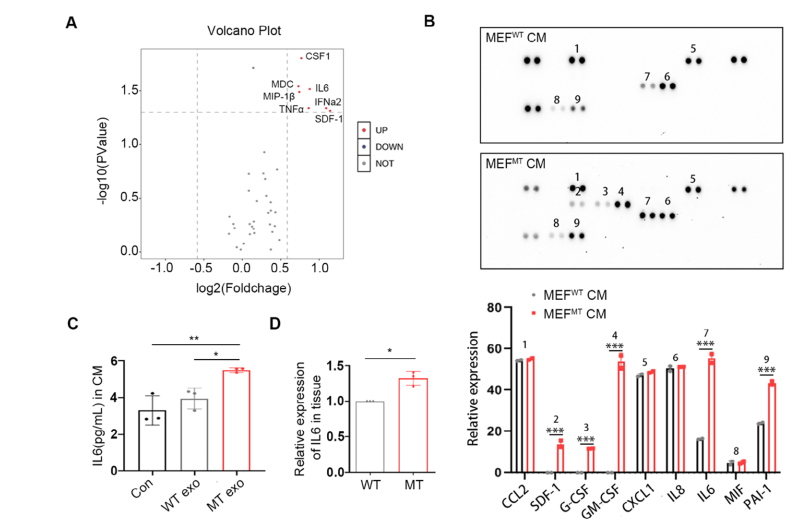
Figure 5. Horizontal Transfer of Exosomal PIK3CA H1047R Mutation Induces CAFs to Secrete Elevated Levels of IL-6
A key function of CAFs is the production of paracrine cytokines, which in turn promote tumor metastasis. The research team analyzed the conditioned medium (CM) from MEF cells treated with WT- or MT-cell-derived exosomes using Olink secretome profiling and a cytokine array system, revealing a significant increase in IL-6 levels. These results were further validated by ELISA and real-time PCR, confirming the elevated IL-6 secretion induced by MT-cell exosomes.
6.CAFs Promote WT Cell Metastasis via the IL-6/IL-6R/STAT3 Signaling Pathway
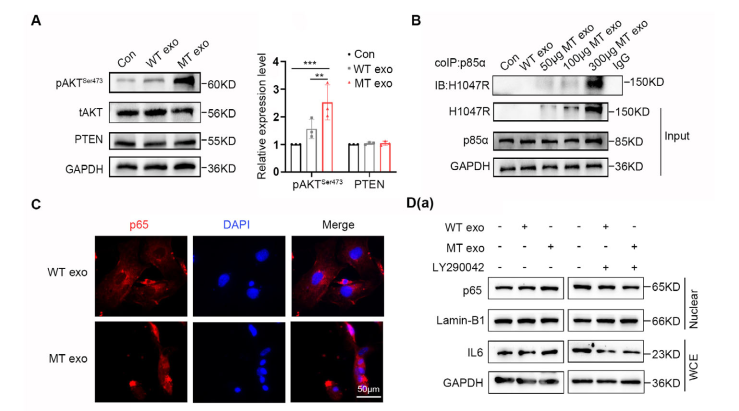
Figure 6. CAFs Promote Colorectal Cancer Metastasis via the IL-6/JAK2/STAT3 Pathway
Further mechanistic studies revealed that horizontal transfer of exosomal PIK3CA DNA to MEF cells activates them into CAFs via the PI3K/NF-κB pathway. These CAFs, in turn, promote the metastasis of WT colorectal cancer cells through the IL-6/JAK2/STAT3 signaling pathway.
Research Significance and Perspectives
Revealing a Unique Phenomenon: This study demonstrates that horizontal transfer of exosomal PIK3CA DNA to fibroblasts activates them into CAFs, which in turn promotes tumor cell metastasis.
Clarifying the Functional Mechanism: The research confirms that horizontal transfer of PIK3CA mutations via CRC-derived exosomes plays a critical role in modulating tumor microenvironment homeostasis and elucidates a potential molecular mechanism underlying CRC metastasis. Furthermore, it highlights that combined detection and targeted intervention of PIK3CA mutations and IL-6 may represent a novel strategy for CRC diagnosis and therapy.
Providing Clinical Insights: The study establishes that serum IL-6 levels in CRC patients correlate with PIK3CA mutation status, and both factors are closely associated with metastatic progression.
- Development of CAF-specific inhibitors targeting PIK3CA mutations (e.g., neutralizing antibodies or small-molecule inhibitors) to evaluate their therapeutic potential in CRC.
- Elucidation of the precise mechanisms by which exosomal DNA is delivered to recipient cells.
Future Directions:
This study not only provides a new perspective on the mechanisms of tumor metastasis but also opens up novel avenues for early detection and targeted therapy of colorectal cancer.
Support Provided by Ubigene
In the study led by Prof. Fang Jin and published in Advanced Science, Ubigene provided the LS174T cell model carrying PIK3CA point mutations, which played a crucial role in elucidating the horizontal transfer of exosomal PIK3CA mutations within the tumor microenvironment.
For those seeking a fully hands-off solution, Ubigene provides a complete point-mutation cell construction service, with pricing starting as low as $6480, enabling scientists to focus on their core research while significantly improving experimental efficiency.
Contact us to get more information >>>Reference
Wang.R, Li, WM., lV, YQ. et al. Colorectal Cancer Cells–Derived Exosomal PIK3CA Mutation DNA Promotes Tumor Metastasis by Activating Fibroblast and Affecting Tumor Metastatic Microenvironment. Advanced Science 12,27 (2025). https://doi.org/10.1002/advs.202501792


