Expert Insights | HEI-OC1 Cell Culture and Gene-Editing Guidelines


Expert Insights | HEI-OC1 Cell Culture and Gene-Editing Guidelines

HEI-OC1 cell is a classic auditory research model derived from mouse cochlear hair cells. They are widely used in studies of hearing loss mechanisms, ototoxic drug screening, and the development of hearing protective drugs. This cell line has stable expression of hair cell markers, is easy to culture and transfect, and serves as an essential tool for exploring auditory biology and developing related therapeutic strategies. It is widely applicable in otolaryngology research, pharmaceutical drug screening, and gene therapy research. In this guide, we will provide a detailed overview of the HEI-OC1 cell line, which has become a prominent cell line in auditory research.
Order HEI-OC1 cell now>>>Cell Information
Cell Name: HEI-OC1 (Mouse Cochlear Hair Cell Line)
Cell Morphology: Epithelial-like, adherent
Cell Culture Conditions: DMEM + 10% FBS
Gas Phase: Air: 95%; CO2: 5%
Temperature: 37°C (±0.5 °C)
Media Change Frequency: Every 2-3 days
Passage Ratio: 1:3 - 1:5
Note: HEI-OC1 cells are temperature-sensitive. At 33 °C they proliferate rapidly and dedifferentiate; at 39 °C proliferation is suppressed and differentiation is induced.
Microscopic morphology
Normal: polygonal, cobblestone-like monolayer with clear borders.
A.
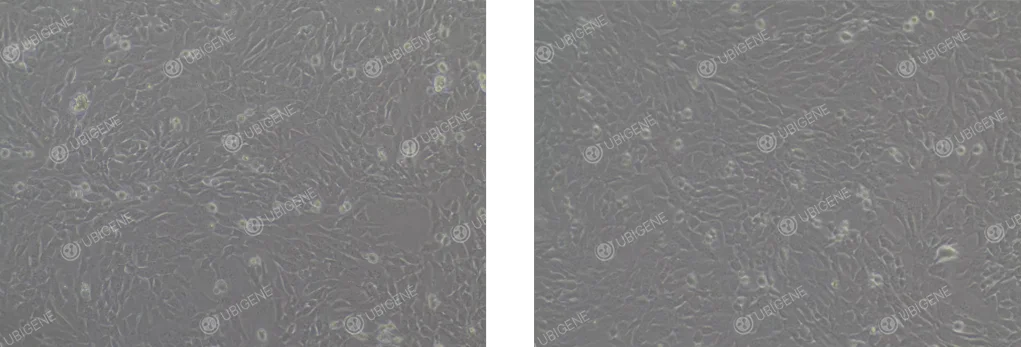
Poor: irregular shape, blurred edges, cytoplasmic granules, low refractive index.
Poor Cell Growth Image: Cell morphology changes, with cells becoming rounder and more floating. The cytoplasm may vacuolate, and cells may collapse, losing their three-dimensional structure; signs of aging are present
B.
Figure 1. Typical morphology of HEI-OC1 cells under normal (A) and stressed (B) conditions.
Cell Thawing
1)Preparation: Equilibrate 7 mL complete medium in a 15 mL tube.
2)Thawing: Thaw the vial in a 37 °C water bath for ~1 min (lid above water level) until only a pea-sized ice crystal remains.
3)Centrifugation: Transfer contents to the tube; centrifuge at 1100 rpm, 4 min; discard supernatant.
4)Resuspension: Resuspend in fresh medium and seed into culture vessels.
5)Culturing: Incubate at 37 °C; check attachment after 24 h.
Cell Passaging (Using T25 flasks as an example)
1)At 70–80% confluence, remove medium and wash twice with 5 mL PBS.
2)Add 1 mL trypsin, tilt to cover the monolayer; incubate 1-2 min until most cells round up.
3)Neutralize with 2 mL complete medium, collect cells into a 15 mL tube.
4)Centrifuge 1100 rpm, 4 min; resuspend in fresh medium.
5)Seed at 1:3–1:5; inspect the next day.
5)Passage the cells in a ratio of 1:2 to 1:4, and observe the cell condition the next day.
Cell Cryopreservation
1)Cell Collection: Harvest log-phase cells as above.
2)Centrifugation: Centrifuge 1100 rpm, 4 min; discard supernatant.
3)Resuspend in freezing medium at 1 × 10⁶ cells/mL; aliquot into labeled cryovials.
4)Use a controlled-rate freezing container; store at –80 °C overnight, then transfer to liquid nitrogen.
General Suggestions for Adjusting Cell Conditions
1)Culture Medium and Serum: DMEM + 10 % FBS, 4 °C, use within 2 weeks.
2)Culture Environment: 37 °C, 5 % CO₂, ≥90 % humidity.
3)Avoid using expired or long-stored media: Passage at 70–80 % confluence to avoid spontaneous differentiation.
4)Cell Passaging Procedures: Avoid over-trypsinization.
5)Maintain master and working cell banks; discard cultures beyond passage 30.
Suggestions for for HEI-OC1 Cell Transfection
Use cells at 70–80 % confluence in log phase, viability ≥90 %. Determine optimal antibiotic concentration beforehand.
Electroporation
- • Control cell number; post-pulse plating density should yield ≥70 % attachment.
- • Minimize handling time.
Lentiviral transduction
- • Pilot MOI optimization.
- • Infect at 30–40 % confluence in the presence of 8 µg/mL Polybrene.
Lipid-mediated transfection
- • Select reagents validated for HEI-OC1.
- • Plate cells 24 h before transfection at 60–70 % confluence.
Suggestions for EO771 Single-cell Cloning Experiment
• Use log-phase cells at ~70 % confluence; viability ≥85 %.
• Pre-warm all reagents.
• Determine limiting dilution empirically (target 1–2 cells/well).
• Seed 96-well plates under sterile conditions to ensure even distribution.
Image of Cells After Lentiviral Infection
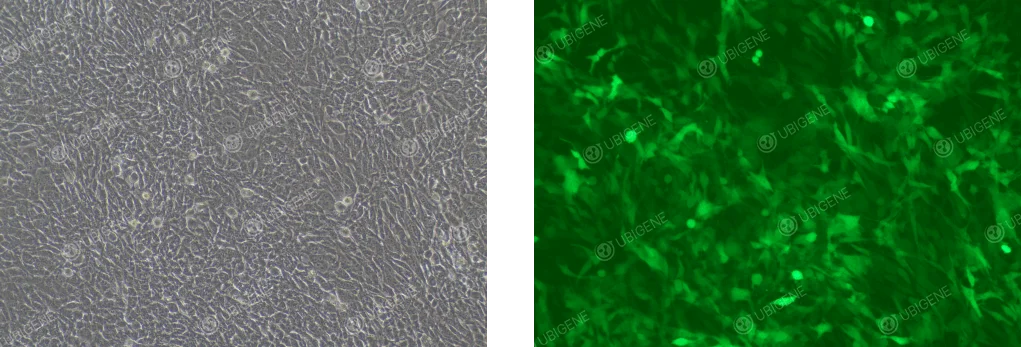
Figure 2. Typical image of lentivirus transfection
Any more questions? Feel free to inquire>>>


