Practical HuH-7 Cell Culture and Gene-Editing Protocols


Expert Insight | Practical HuH-7 Cell Culture and Gene-Editing Protocols
The HuH7 cell line originates from human hepatocellular carcinoma and was established in 1982 by Japanese researchers using liver tumor tissue from a 57-year-old male patient. Because it retains several key hepatocyte functions—such as albumin synthesis—and is straightforward to maintain under in vitro conditions, HuH7 has become widely utilized in studies of liver disease pathogenesis, HCV/HBV replication biology, drug discovery, and tumor research. Today, it is regarded as a classic and indispensable cellular model for both fundamental and applied liver-related research. To support your work with this widely used cell line, Ubigene is pleased to share an exclusive set of insights to help you further explore best practices in HuH-7 cell culture and gene-editing applications.
I. Overview of Human Hepatoma Cell Line (HuH-7)
Cell Name: Human Hepatoma Cell Line (HuH-7)
Morphology: Epithelial-like, adherent growth
Culture Medium: 90% DMEM + 10% FBS
Gas Phase: 95% air; 5% CO₂
Temperature: 37 °C
Medium Renewal Frequency: Every 2–3 days
Subculture Ratio: 1:2 - 1:3
Reference for Cell Growth Status:
● Normal Morphology: Under normal conditions, HuH-7 cells typically exhibit an epithelial, polygonal appearance. Most cells present as irregularly shaped polygons, although a subset may appear elongated or spindle-like.
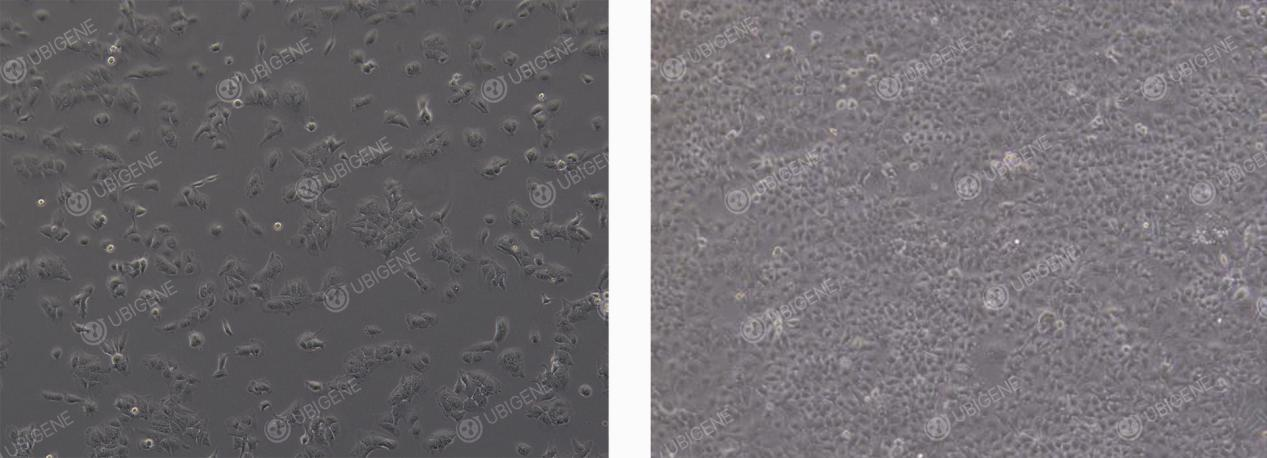
HuH-7 Normal Morphology
● Abnormal Morphology: In abnormal conditions, cells may appear contracted and rounded with weakened adherence; some cells may exhibit elongated, unhealthy filopodia-like extensions; an increase in cellular debris or the presence of clear vacuoles within the cytoplasm may also be observed.
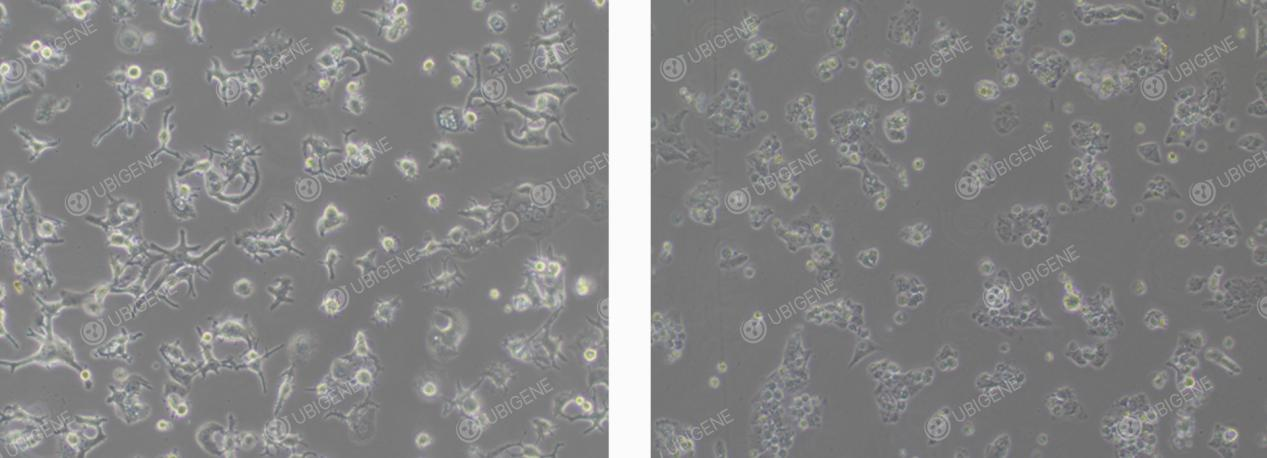
HuH-7 Abnormal Morphology
II. HuH-7 Cell Thawing Procedure
1. Preparation of Culture Medium
Aliquot 7 mL of complete culture medium into a sterile centrifuge tube and set aside.
2. Cell Thawing
Remove the cells from dry ice. Hold the vial cap with sterile forceps and immerse the vial in a 37°C water bath, gently swirling (Important: do not allow water to cover the cap). Thaw for approximately 1 minute until the ice block melts to the size of a mung bean, then immediately remove from the water bath.
3. Cell Centrifugation
Transfer the thawed cell suspension into a centrifuge tube and centrifuge at 1100 rpm for 4 minutes. Carefully discard the supernatant.
4. Resuspension and Seeding
Resuspend the cell pellet in complete culture medium and seed into an appropriately sized culture dish or flask.
5. Continued Culture
Place the culture dish or flask in a 37°C incubator. After 24 hours, assess cell morphology and adhesion.
III. HuH-7 Cell Passaging (e.g., T25 Flask)
1. Subculture Conditions
● Subculture when cells reach 80–90% confluency.
● In a biosafety cabinet, remove the culture medium from the flask and wash the cells 1–2 times with 5 mL of PBS.
2. Trypsinization
● Add 1 mL of trypsin to the flask. Gently swirl to ensure that the trypsin fully covers the cell layer.
● Incubate the flask in a 37°C incubator for 1–2 minutes.
● Monitor cells under a microscope. When most cells become rounded and bright, and detach easily upon gentle agitation, immediately stop the digestion.
3. Termination of Digestion
● Add 2 volumes of complete culture medium (2 mL) to neutralize the trypsin.
● Transfer the cell suspension into a 15 mL centrifuge tube.
4. Cell Centrifugation
● Centrifuge at 1100 rpm at room temperature for 4 minutes.
● Carefully discard the supernatant.
● Resuspend the cell pellet in complete culture medium.
5. Cell Passaging
● Seed cells into new culture flasks at a split ratio of 1:2 to 1:3.
● Assess cell morphology and attachment the following day.
IV. HuH-7 Cell Cryopreservation Procedure
1. Cell Collection
Collect the trypsinized cells according to standard subculture procedures and transfer them into a centrifuge tube.
2. Cell Centrifugation
Centrifuge the cell suspension at 1100 rpm for 4 minutes. Carefully discard the supernatant.
3. Resuspension and Freezing
● Resuspend the cell pellet in cryopreservation medium, adjusting the concentration to 1 × 10^6 cells/mL.Aliquot 1 mL of the cell suspension per cryovial.
● Label each vial with cell line name, passage number, and date.
4. Cooling and Storage
Place the cryovials in a controlled-rate freezing container and store at −80°C overnight. Transfer the vials to a liquid nitrogen tank for long-term storage.
V. HuH-7 Cell Culture Considerations
1.Cell Morphology: HuH-7 cells exhibit multiple protrusions/pseudopodia, which is a normal characteristic.
2.Culture Medium Storage: Ensure that the culture medium is stored at 4°C, protected from light, and used within its expiration date.
3.Culture Environment: Maintain optimal cell culture conditions.(temperature, CO₂ concentration, humidity)
4.Operational Details: Pre-warm culture medium and trypsin to 37°C before use to avoid temperature-induced stress.
5.Subculture Practices: Avoid excessive pipetting to prevent damage to the cell membrane; if cells are unevenly attached, gently swirl the flask to distribute cells evenly.
VI. HuH-7 Cell Transfection Considerations
1. Cell Condition Requirements
● Ensure cells are healthy and in the logarithmic growth phase, with 70–80% confluency.
● Cell viability >85%, as determined by Trypan Blue staining.
● Use low-passage cells whenever possible.
● Monitor trypsinization time carefully to avoid over-digestion and cellular damage.
● During handling, dissociate cells into a single-cell suspension to prevent clumping.
2. Transfection Reagents and Preliminary Experiments
● Thoroughly mix transfection reagents before use to ensure homogeneity.
● It is recommended to perform preliminary drug selection experiments to determine the optimal selection concentration post-transfection.
3. Electroporation Transfection
● Control the number of cells; seed the electroporated cells into appropriately sized culture plates according to the cell quantity.
● Use mild trypsin and completely neutralize with serum-containing medium.
● Wash cells 1–2 times with PBS to remove residual serum, preventing ionic interference during electroporation.
● Perform preliminary experiments to optimize electroporation parameters.
● Ensure post-electroporation cell attachment rate ≥70%.
● Keep total electroporation time minimal to avoid excessive stress.
4. Lentiviral Transduction
● Perform preliminary experiments to determine the optimal MOI(multiplicity of infection).
● Control cell confluency at 30–40% before infection; do not exceed this range.
● Add Polybrene prior to infection to enhance efficiency.
● Change medium 24 hours post-infection.
● Avoid repeated freeze-thaw cycles of the viral stock.
● If infection efficiency is low, consider reinfection (ensure cells tolerate virus well) or try centrifugal infection.
5. Lipid-Based Transfection
● Choose lipid transfection reagents optimized for HuH-7 cells.
● Optimize DNA-to-reagent ratios via preliminary experiments to identify the best transfection conditions.
● Use provided enhancers if available, which can significantly improve efficiency in difficult-to-transfect cells.
● Plate cells 24 hours before transfection; target 60–70% confluency at the time of transfection.
● Dilute DNA in Opti-MEM first, then add lipid reagent (reverse order will reduce efficiency).
● Allow complexes to incubate at room temperature for 15–20 minutes (less than 10 minutes → incomplete complex formation; more than 30 minutes → increased toxicity).
● Do not include antibiotics in transfection medium, as cationic lipids increase membrane permeability, potentially causing antibiotic toxicity.
● Add complexes slowly and evenly to the culture medium, gently swirling to mix; avoid direct impact or vigorous pipetting to prevent cell damage.
VII. HuH-7 Single-Cell Cloning (Clone Screening) Considerations
1. Cell Condition Requirements
● Use cells in the logarithmic growth phase for single-cell cloning. Prior to seeding, maintain cell confluency at approximately 70%.
● Cell viability should be ≥80% at the time of seeding.
2. Reagents and Preliminary Preparation
● Pre-warm all reagents (including culture medium, trypsin, and PBS) before use.
● It is recommended to use gentle dissociation reagents (such as TrypLE Express).
3. Single-cell cloning Strategy
● Perform preliminary experiments to determine the optimal seeding density for single-cell cloning, ensuring an appropriate proportion of single clones.
● When seeding into 96-well plates, ensure even distribution of cells; add PBS to outer wells to prevent evaporation.
4. Dilution Method
● Use the “limiting dilution method” for single-cell cloning.
● After cell counting and dilution, aim for a final concentration of 1 × 10^6 – 2 × 10^6 cells/mL.
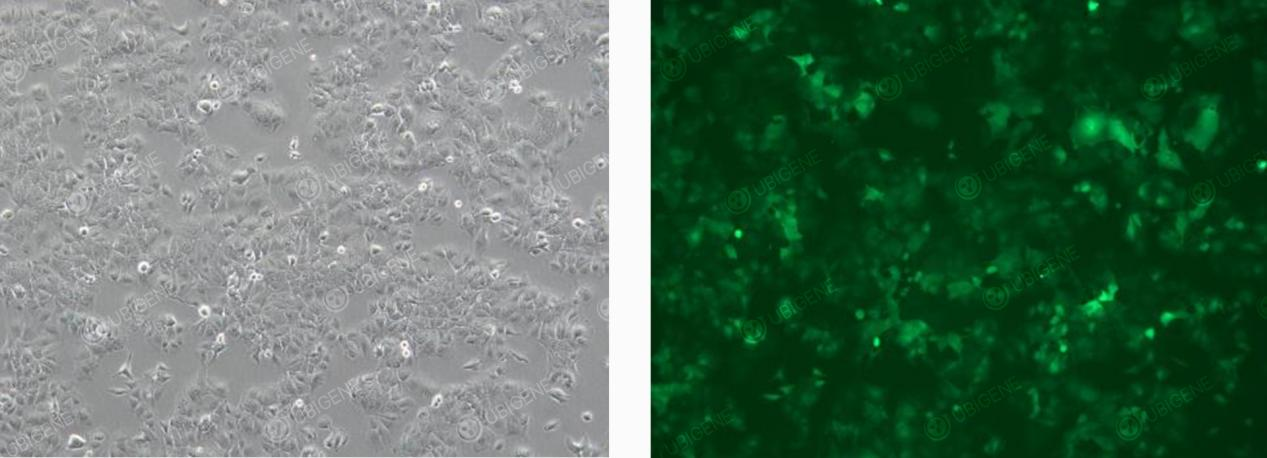
Cells Following Lentiviral Transduction
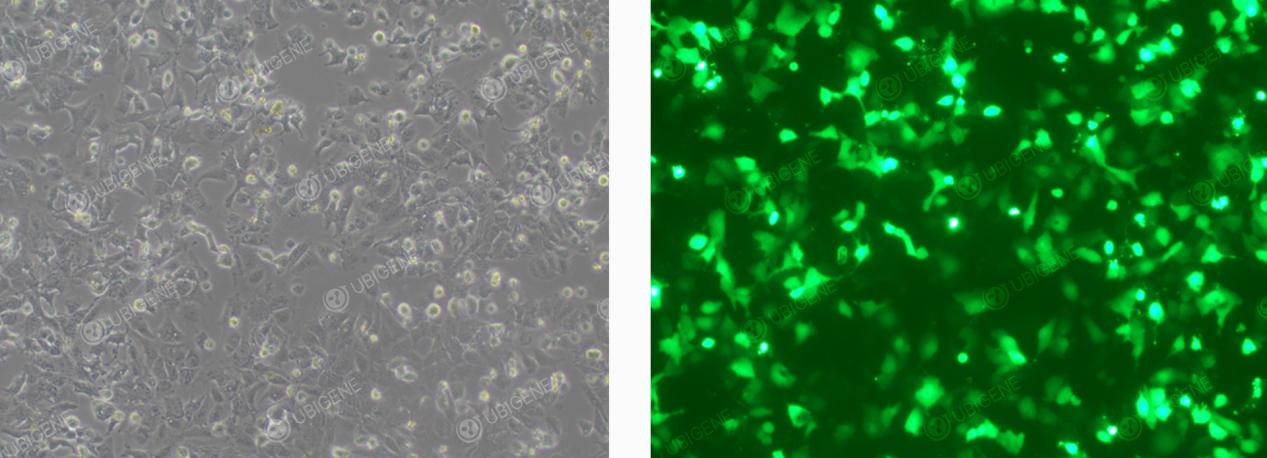
Cells Following Electroporation Transfection
VIII. HuH-7 Cell Culture Common Issues and Solutions
1. Slow Cell Growth
Observation: Cell proliferation is significantly slower than normal (normal subculture interval: every 2–3 days).
Possible Causes:
(1) Excessive passage number: cell senescence and reduced viability.
(2) Seeding density too low: cells cannot reach optimal confluency for healthy growth.
(3) Suboptimal culture conditions: incubator temperature, CO₂ concentration, or humidity inaccurate or unstable.
Solutions:
(1) Limit passage number: avoid over-passaging and subculture cells during logarithmic growth phase.
(2) Adjust seeding density: seed cells within an optimal range based on growth characteristics.
(3) Check incubator parameters: ensure temperature, CO₂ concentration, and humidity are correct and stable.
2. Cell Clumping and Uneven Distribution
Observation: Cells form irregular clumps after subculture; they may aggregate at the center or edges of the flask.
Possible Causes:
(1) Inadequate pipetting to dissociate cells;
(2) Cells not evenly resuspended before seeding.
Solutions:
(1) Gently increase pipetting to break up large cell clumps without damaging cell membranes.
(2) After seeding, gently swirl the flask using “cross” or “figure-eight” motions to evenly distribute cells.
3. Abnormal Cell Morphology
Observation: Cells appear shrunken with unclear edges; some cells enlarge, show increased granularity, or vacuolation.
Possible Causes:
(1) Poor serum quality or aged cells;
(2) Nutrient deficiency or over-trypsinization;
(3) Accumulation of metabolic waste from prolonged culture.
Solutions:
(1) Use high-quality fetal bovine serum (FBS); thaw and use low-passage frozen cells.
(2) Replace with fresh culture medium; adjust trypsin digestion time (generally 1–2 minutes).
(3) Subculture and change medium in a timely manner; do not wait until cells reach 100% confluency—passage at 80–90% confluency for optimal health.
Pro Tip: When observing HuH-7 cells under the microscope, the presence of multiple protrusions or pseudopodia is normal. However, excessively elongated, unhealthy filamentous pseudopodia indicate abnormal cell conditions and should be distinguished carefully.
If you’re planning to use HuH-7 cells for hepatocellular carcinoma research, Ubigene’s Red cotton OmniCell bank offers over 1,000 wild-type cell lines, Click here to find your target cell line>>



