iPS Knockout Cell Lines: A Comprehensive Guide
Mastering the Use of iPS Knockout Cell Lines to Build a High-Scoring Research System: A Comprehensive Guide

Induced pluripotent stem cells (iPSCs) are a type of stem cells that are artificially induced from somatic cells through specific methods, reversing their developmental state to regain pluripotency similar to that of embryonic stem cells. iPSCs possess the ability to self-renew and differentiate into multiple cell lineages, making them one of the core focuses in stem cell biology and regenerative medicine. In traditional medicine, when facing medical challenges such as organ failure and neurodegenerative diseases, treatments often rely on allogeneic cell transplantation. However, due to limited donor availability and the risk of immune rejection, these approaches rarely achieve ideal therapeutic outcomes. Moreover, the lack of disease models means that traditional medicine can only focus on "intervention after disease onset," making it difficult to elucidate pathogenic mechanisms and develop targeted drugs for precision medicine. The advent and development of iPSC technology have brought endless possibilities to the current medical dilemmas, with the following core advantages:
1.Solving the donor source problem: The initial cells for iPSCs can be easily obtained from donors' skin, blood, and urine samples. Once induced, iPSCs have the ability to proliferate long-term, providing sufficient cells for downstream experiments or differentiation into functional cells.
2.Eliminating the risk of immune rejection: iPSCs can be derived from a patient's own somatic cells, enabling personalized autologous cell therapy and completely avoiding the risk of immune rejection associated with allogeneic transplantation.
3.Reducing ethical controversies: The acquisition of cells does not involve embryo manipulation, thereby resolving ethical disputes and aligning with the moral standards of modern scientific development.
![Figure 1. The pluripotency of iPSCs [1]](/uploads/allimg/250428/pluripotency-of-ipscs.webp)
Figure 1. The pluripotency of iPSCs [1]
iPSCs and Gene Editing
The CRISPR/Cas9 system has become the most widely used gene-editing tool in the life sciences due to its efficiency, simplicity, and low cost. By precisely targeting the genome with its key components, sgRNA and Cas9 protein, and combining with cellular repair mechanisms, the CRISPR/Cas9 system can achieve precise gene editing in cells and organisms, including gene knockout, base substitution, and gene knock-in. In addition to the aforementioned core advantages, iPSCs, with their pluripotency, can be effectively applied in research areas such as disease mechanism exploration, drug development, and regenerative medicine when combined with CRISPR gene-editing technology. Gene knockout is currently the most commonly used gene-editing method. iPSC gene knockout cell lines have become one of the core research tools in the field of regenerative medicine and have frequently appeared in high-scoring, high-quality papers in recent years. However, after obtaining iPSC gene knockout cell lines, how should they be applied? What research strategies are needed to build our research system and stand out in the highly competitive scientific research community? This article will introduce some "advanced" applications of iPSC knockout cell lines, analyzing how to use them to elevate our research system and publish high-impact papers.
Applications of iPSC Knockout Cell Lines
1.Embryonic Development Mechanism Research
iPSCs have the potential to differentiate into various adult cell lineages under the influence of specific factors. Therefore, human iPSCs can effectively simulate the early embryonic development process of the human body and have been widely used to study tissue and organ regeneration and repair, as well as the molecular mechanisms of early cell fate determination. They are one of the best in vitro cell models for studying human development.
Representative Article: Epitranscriptomic regulation of cortical neurogenesis via Mettl8-dependent mitochondrial tRNA m3C modification,Cell Stem Cell, IF=19.8
In this study, the research team led by Professors Guoli Ming and Hongjun Song from the University of Pennsylvania used mouse models and human iPSC-derived forebrain organoids to successfully reveal the important function of mitochondrial tRNA m3C modification and its methyltransferase METTL8 in regulating neurogenesis. This study unlocked a new mechanism of epitranscriptomic regulation in cortical development. Based on clues obtained from Mettl8 knockout conditional mice in the early stages of the study, the authors further constructed a METTL8 knockout cell line in human iPSCs and differentiated both wild-type and iPSC knockout cell lines into human forebrain organoids. During this process, they found phenotypes consistent with those in the mouse model. The down-regulation of m3C modification levels of mitochondrial tRNAThr/Ser(UCN) caused by METTL8 deficiency similarly led to severe impairment of mitochondrial protein translation in neural stem cells of human forebrain organoids, resulting in the depletion of neural stem cells. Although RNA chemical modifications have been shown to play key roles in many important biological processes and pathological conditions, current research is still focused on popular modifications such as m6A, while the functions and mechanisms of other types of modifications remain to be elucidated. In this study, the authors combined mouse models, iPSC knockout cell lines, and human forebrain organoid differentiation to fully clarify the specific regulatory roles of METTL8 and its mediated mt-RNA m3C modification in neural stem cell mitochondrial protein translation and cortical development. The findings were published in the top journal in stem cell biology, Cell Stem Cell[2].
![Figure 2. iPSC knockout cell line reveals a new mechanism of epigenetic transcriptional regulation in cortical development [2]](/uploads/allimg/250428/ipsc-knockout-cell-line-epigenetic-transcriptional-regulation.webp)
Figure 2. iPSC knockout cell line reveals a new mechanism of epigenetic transcriptional regulation in cortical development [2]
2.Disease Mechanism Exploration and Drug Development
Generally, the advantage of iPSCs in disease model construction lies in the ability to directly obtain somatic cells from patients and reprogram them into iPSCs that can proliferate indefinitely and have the same genetic background as the patient. However, for diseases with significant individual genetic heterogeneity, reprogramming a large number of different patient somatic cells would undoubtedly increase the experimental and time costs and complicate the process. Therefore, we can also introduce genetic interventions or disease-causing mutations into iPSCs derived from healthy individuals through gene knockout, point mutation, or gene knock-in methods to obtain the corresponding pathological phenotypes and construct disease models.
Representative Article: Commander complex regulates lysosomal function and is implicated in Parkinson's disease risk, Science, IF=44.8
Heterozygous pathogenic variants in the GBA1 gene are a common risk factor for Parkinson's disease (PD) and dementia with Lewy bodies. In some patients, lysosomal homeostasis abnormalities may be closely related to disease mechanisms or progression. In this study, to identify specific genes and cellular pathways that affect GCase activity and lysosomal homeostasis, Dimitri Krainc's team at Northwestern University conducted a genome-wide CRISPR interference screen to identify genetic modifiers of GCase activity and lysosomal function. Based on the screening results, they found that copper metabolism MURR1 domain protein 3 (COMMD3) and its associated COMMD/CCDC22/CCDC93 (CCC) and Commander complexes are potential regulators of GCase activity and lysosomal function. In subsequent functional validation, the authors knocked out COMMD3 in iPSCs and differentiated them into dopaminergic neurons. COMMD3 knockout led to decreased GCase activity and α-synuclein aggregation, mimicking key phenotypes of PD and serving as a disease model to deeply explore the role mechanism of COMMD3 in the human system. This article successfully revealed the central role of the Commander complex in lysosomal function and its mechanism as a PD risk factor, providing a new direction for the treatment of neurodegenerative diseases. The study was published in the top academic journal Science[3].
![Figure 3. Dopaminergic neuron differentiation of iPSC knockout cell lines to simulate PD phenotypes [3]]( /uploads/allimg/250428/dopaminergic-neuron-differentiation-ipsc-knockout-pd-phenotypes.webp)
Figure 3. Dopaminergic neuron differentiation of iPSC knockout cell lines to simulate PD phenotypes [3]
3.Engineering Functional Cells
The multilineage differentiation potential of iPSCs allows them to differentiate into specific functional cells, such as pancreatic β-cells, dopaminergic neurons, and immune cells, which can be used for the production of functional secretory proteins and tumor immunotherapy. However, these iPSC-derived functional cells often face certain challenges in use. For example, iPSC-derived natural killer (iNK) cells have difficulty achieving good therapeutic effects in the treatment of relapsed and refractory acute myeloid leukemia, with few patients achieving complete remission. Therefore, additional engineering modifications are needed to enhance the efficacy of these iNK cells, and the most commonly used method is genetic intervention, such as gene knockout.
Representative Article: Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy,Cell Stem Cell, IF=19.8
In this study, the teams of Frank Cichocki and Jeffrey S.Miller from the Department of Medicine at the University of Minnesota in the United States developed an engineered iNK cell product through gene editing. The engineered iNK cells can persist and function in vivo even in the absence of exogenous cytokines and can be combined with therapeutic antibodies to enhance tumor targeting. Based on the hnCD16-iPS cell line established in previous studies, the authors further knocked out CD38 in this cell line to achieve high levels of NAD+and enhanced resistance to oxidative stress, while also avoiding NK cell fratricide mediated by the therapeutic anti-CD38 antibody daratumumab. Mass spectrometry analysis revealed that CD38 knockout mainly affected glycolysis and cysteine metabolism,leading to increased intracellular levels of cysteine-glutathione disulfide (L-CySSG) and enhanced resistance to oxidative stress in these cells. Additionally, the authors introduced an IL-15/IL-15R fusion protein to further improve the functionality and signaling durability of the cells. Ultimately, the authors named this highly effective engineered cell product “iADAPT” NK, and the research findings were successfully published in the top journal in stem cell biology, Cell Stem Cell[4].
![Figure 4. Genetic knockout and other editing methods for engineering NK cells [4]](/uploads/allimg/250428/genetic-knockout-editing-methods-for-nk-cells.webp)
Figure 4. Genetic knockout and other editing methods for engineering NK cells [4]
4.Personalized Cell Therapy
iPSCs derived from patients carry the same genetic background as the patients themselves. Combined with in vitro differentiation, they can perfectly replicate the true physiological characteristics of diseases. Depending on the different pathological mechanisms of various diseases, we can use gene-editing methods to perform gene knockout or gene repair on patient-derived iPSCs, restoring their normal physiological functions and making them a potential source of cells for personalized transplant therapy.
Representative Articles:
- 1. CCR5 disruption in induced pluripotent stem cells using CRISPR/Cas9 provides selective resistance of immune cells to CCR5-tropic HIV-1 virus,Molecular Therapy-Nucleic Acids, IF=6.5
- 2. CRISPR-Edited Stem Cells in a Patient with HIV and Acute Lymphocytic Leukemia, The new england journal of medicine, IF=96.3
Chemokine receptor 5 (CCR5) serves as a co-receptor for HIV virus, playing a crucial role in the infection of CCR5-tropic virus strains. Studies have shown that functional loss of CCR5 can prevent HIV infection. In 2015, Igor I Slukvin's research team published work in Molecular Therapy-Nucleic Acids where they used gene knockout to obtain CCR5 knockout homozygous iPSC clones [5]. These cell lines could effectively differentiate into hematopoietic cells and macrophages, and exhibited unique resistance to CCR5-tropic viruses in in vitro HIV infection experiments. Building on this research, a clinical trial was conducted by a joint team from Peking University (Hongkui Deng), the 307 Hospital of the Chinese People's Liberation Army (Hu Chen), and Beijing You'an Hospital (Hao Wu). They performed CCR5 gene knockout on adult human hematopoietic stem cells. These edited stem cells achieved long-term stable hematopoietic system reconstruction in the human body and were used to treat a 27-year-old male patient with HIV and acute lymphocytic leukemia. After treatment, the patient's acute lymphocytic leukemia achieved complete remission, and his T cells showed some resistance to HIV. This clinically significant study was published in the top medical journal The New England Journal of Medicine[6].
![Figure 5. Construction of CCR5 knockout iPSC lines (left); therapeutic outcomes after CCR5 knockout stem cell transplantation (right) [6].](/uploads/allimg/250428/ccr5-knockout-ipsc-lines-therapeutic-outcomes.webp)
Figure 5. Construction of CCR5 knockout iPSC lines (left); therapeutic outcomes after CCR5 knockout stem cell transplantation (right) [6].
The above analysis of the applications of iPSC knockout cell lines in high-impact articles may have already inspired you with ideas for designing iPSC-related research topics and made you eager to get started. However, despite the great potential of iPSCs, compared to other commonly used tumor cell lines, there are still significant challenges in gene editing of iPSCs. Obtaining successfully edited cell lines is not easy.
Challenges in Gene Knockout of iPSCs
1.Maintenance of Pluripotency and Cell State: iPSCs are relatively "delicate" and have very strict culture requirements. A complete culture system is needed to maintain the pluripotency and self-renewal ability of iPSCs. Changes in cell state or differentiation can severely affect the editing efficiency and long-term maintenance of iPSCs. Physical stimuli and external shear forces during the gene-editing process can also lead to deterioration of cell state or differentiation, significantly impacting the efficiency and long-term culture of iPSCs.
2.Low Transfection Efficiency: iPSCs are difficult to transfect. Commonly used chemical transfection methods, such as Lipo3000 and PEI, have poor transfection efficiency in human pluripotent stem cells. Electroporation (electroporation) is more commonly used for iPSC transfection, but the conditions and density for electroporation need to be precisely optimized.
3.Difficulty in Single-Clone Formation: There are still issues with low survival rates and difficulty in maintaining pluripotency during single-clone culture of iPSCs. Current single-clone acquisition methods generally rely on cloning techniques outside the laminar flow hood, which carry a higher risk of contamination.
Construction of Gene Knockout iPSC Lines
The most commonly used method for gene knockout is mediated by non-homologous end joining (NHEJ), a DNA double-strand break repair mechanism. After the CRISPR/Cas9 system induces a double-strand break in the genomic DNA through sgRNA and Cas9, the cell initiates a repair program for the double-strand break. NHEJ is the most prevalent mechanism in this process. This mechanism does not require any template and can join the broken ends together with the help of specific repair proteins and DNA ligases, often resulting in random insertions or deletions of bases. These insertions or deletions usually cause frameshift mutations, leading to the absence of normal protein encoding and thus achieving the effect of gene knockout.
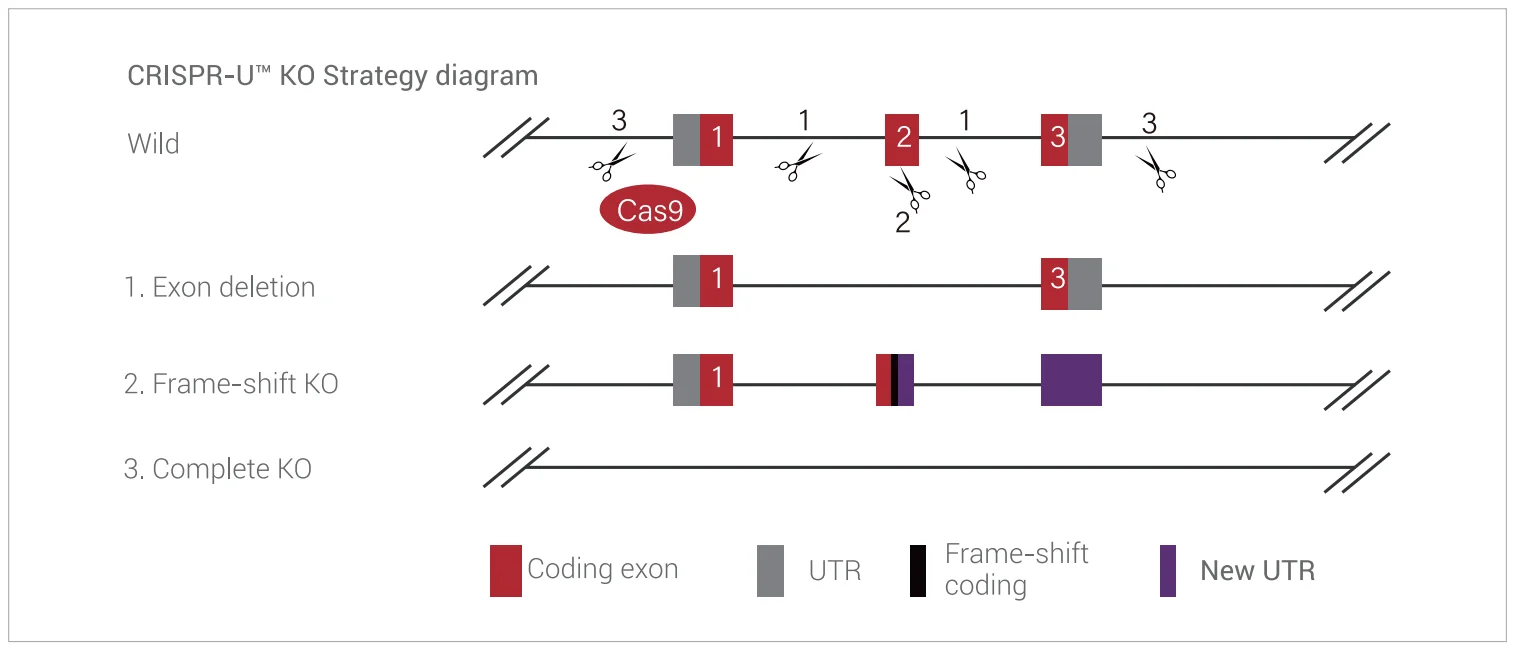
Figure 6. Principle of constructing iPSC knockout cell lines based on the NHEJ mechanism.
Ubigene's CRISPR-U™ technology, based on the CRISPR system and NHEJ repair mechanism, optimizes and controls each process node to maximize the editing efficiency of the target gene in iPSCs, increasing the gene-editing efficiency of iPSCs by 10-20 times. The construction process is as follows:

Figure 7. Construction process of iPSC gene knockout cell lines
Core Advantages of CRISPR-U™ Technology in iPSC Gene Knockout
Based on its independently developed human pluripotent stem cell culture system and CRISPR-U™ technology platform, Ubigene effectively addresses technical challenges such as maintaining the pluripotency of iPSCs, improving transfection efficiency, and obtaining single clones.
1.Independently Developed Human Pluripotent Stem Cell Culture System:
Through independent research and development, Ubigene has optimized the culture conditions for human pluripotent stem cells and successfully established the stable and mature EZ-Stem™ human pluripotent stem cell culture system. This system significantly improves the survival rate, pluripotency maintenance, and single-clone formation rate of human iPSCs during the entire editing cycle. It has also successfully established a human pluripotent stem cell bank (hESCs and hiPSCs) and has extensive experience in stem cell editing.
2.Red Cotton Automated Editing Scheme Design System:
Ubigene's independently developed Red Cotton gene-editing system covers the automated design of gene-editing schemes, gene expression queries, lethality checks, and genotype analysis. It minimizes design errors and subjectivity, ensuring the success rate and efficiency of gene editing.
3.High-Efficiency Acquisition of Positive Single Clones:
With extensive R&D experience and the support of the EZ-Stem™ human pluripotent stem cell culture system, Ubigene has successfully implemented single-clone passaging and separation of human pluripotent stem cells through limiting dilution. This method significantly improves the survival rate and purity of iPSC single clones while avoiding contamination and cross-contamination risks associated with manual cloning operations. Additionally, a rapid single-clone identification system has been developed to genotype cells at an early stage using a non-extraction method, reducing the need for excessive passaging and long-term culture of edited human embryonic stem cells.
4.Comprehensive Pluripotency Detection Methods:
Ubigene's pluripotency assessment system comprehensively covers the detection of pluripotency and multilineage differentiation potential of human pluripotent stem cells. It strictly controls the pluripotency and quality of iPSCs before and after gene editing. Moreover, parallel gene-editing schemes with multiple replicates can precisely identify phenotypic changes after gene knockout.

Obtaining iPSC knockout cell lines is a time-consuming and labor-intensive process, and culturing iPSCs requires a great deal of effort and attention. Experienced researchers are well aware of these challenges. If you have limited resources or lack confidence in iPSC gene editing, Ubigene can provide customized services for iPSC gene knockout, knock-in, and point mutation. With extensive experience in iPSC gene editing, we ensure the delivery of high-quality iPSC gene-edited cell lines. The EZ-Stem™ culture system easily manages iPSC cell culture, and the CRISPR-U™ technology platform supports the entire iPSC gene-editing process. Contact our technical staff and learn more about our services.
References
[1]akahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25.
[2]Zhang F, Yoon K, Zhang DY, Kim NS, Ming GL, Song H. Epitranscriptomic regulation of cortical neurogenesis via Mettl8-dependent mitochondrial tRNA m3C modification. Cell Stem Cell. 2023 Mar 2;30(3):300-311.e11.
[3]Minakaki G, Safren N, Bustos BI, Lubbe SJ, Mencacci NE, Krainc D. Commander complex regulates lysosomal function and is implicated in Parkinson's disease risk. Science. 2025 Apr 11;388(6743):204-211.
[4]Woan KV, Kim H, Bjordahl R, Davis ZB, Gaidarova S, Goulding J, Hancock B, Mahmood S, Abujarour R, Wang H, Tuininga K, Zhang B, Wu CY, Kodal B, Khaw M, Bendzick L, Rogers P, Ge MQ, Bonello G, Meza M, Felices M, Huffman J, Dailey T, Lee TT, Walcheck B, Malmberg KJ, Blazar BR, Bryceson YT, Valamehr B, Miller JS, Cichocki F. Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell. 2021 Dec 2;28(12):2062-2075.e5.
[5]Kang H, Minder P, Park MA, Mesquitta WT, Torbett BE, Slukvin II. CCR5 Disruption in Induced Pluripotent Stem Cells Using CRISPR/Cas9 Provides Selective Resistance of Immune Cells to CCR5-tropic HIV-1 Virus. Mol Ther Nucleic Acids. 2015 Dec 15;4:e268.
[6]Xu L, Wang J, Liu Y, Xie L, Su B, Mou D, Wang L, Liu T, Wang X, Zhang B, Zhao L, Hu L, Ning H, Zhang Y, Deng K, Liu L, Lu X, Zhang T, Xu J, Li C, Wu H, Deng H, Chen H. CRISPR-Edited Stem Cells in a Patient with HIV and Acute Lymphocytic Leukemia. N Engl J Med. 2019 Sep 26;381(13):1240-1247.


