Expert Insights | Practical MC38 Cell Culture and Gene-Editing Protocols


Expert Insights | Practical MC38 Cell Culture and Gene-Editing Protocols
Mouse Colon Carcinoma Cell Line(MC38)—a gold-standard model in tumor immunology. Widely recognized for its high immunogenicity and rapid tumor-forming capacity, MC38 cell line has become an indispensable preclinical model for evaluating PD-1/PD-L1 immune checkpoint inhibitors, advancing cancer vaccine and cell therapy development, dissecting the tumor microenvironment, and screening novel anticancer agents. By offering a robust and reproducible experimental system, MC38 cells empower researchers to accelerate discoveries and strengthen the reliability of their findings. Today, Ubigene provides comprehensive guidance on the essential experimental practices for MC38, including cell thawing, passaging, cryopreservation, transfection, and single-cell cloning. Through a step-by-step approach, we emphasize critical techniques and key precautions to help you minimize experimental risks, streamline workflows, and generate high-quality, reproducible data that drive progress in tumor immunology research.
Overview of Mouse Colon Carcinoma Cells (MC38)
Cell line name: Mouse colon carcinoma cells (MC38)
Morphology: Epithelial-like; mixture of rounded and adherent cells; adherent growth
Culture medium: 90% DMEM + 10% FBS
Gas phase: 95% air, 5% CO₂
Incubation temperature: 37 °C
Medium change frequency: Replace culture medium every 2–3 days
Subculture ratio: 1:2 to 1:4
Reference for MC38 Cell Growth Status
- · Normal morphology: MC38 cells typically appear as irregular polygonal or spindle-shaped cells with well-defined boundaries. They grow as a non-overlapping monolayer with adherent attachment. The cytoplasm is abundant and translucent, reflecting healthy growth (as shown below).
- · Abnormal morphology: Cells exhibit atypical shapes with indistinct edges and reduced cytoplasmic volume or brightness. Optical refraction becomes weaker, and increased cellular debris may be observed. Vacuole formation, abnormal cell enlargement, or flattened morphology indicate compromised growth status (as shown below).

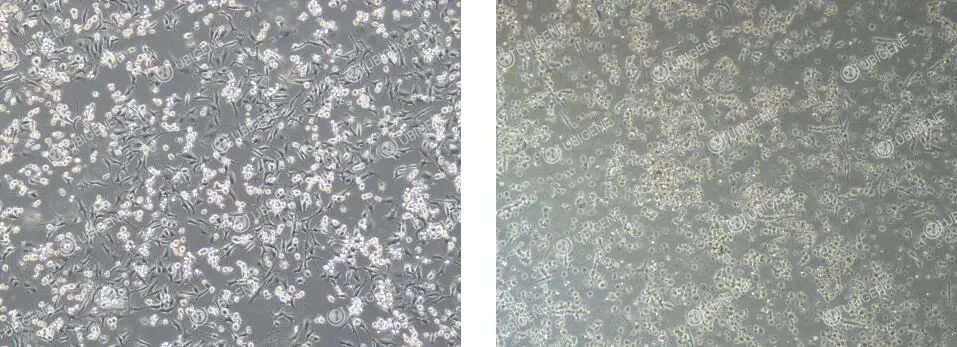
MC38 Cell Thawing Procedure
1. Prepare complete culture medium
Prepare 7 mL of complete medium (DMEM 90% + FBS 10%) in a centrifuge tube and set aside.
2. Cell Thawing
- · Remove the cryovial from dry ice and hold the cap with forceps.Place the vial in a 37 °C water bath and gently swirl.
- · Note: Ensure the water level does not exceed the cap to prevent liquid from entering the vial.
- · Thaw for approximately 1 minute, stopping when only small ice fragments remain.
4. Centrifugation
- · Transfer the thawed cell suspension to a centrifuge tube.Centrifuge at 1100 rpm for 4 minutes.
- · Carefully discard the supernatant without disturbing the cell pellet.
5. Resuspension and seeding
- · Gently resuspend the cell pellet in complete medium until homogeneous.
- · Seed the cells into an appropriately sized culture dish or flask.
6. Culture and monitoring
- · Place the seeded culture dish or flask in a 37 °C, 5% CO₂ incubator.
- · After 24 hours, observe cell attachment and growth to ensure successful recovery.
MC38 Cell Passaging (e.g., T25 Flask)
1. Passaging conditions
- · Passage cells when they reach 80–90% confluence.
- · In a biosafety cabinet, discard the culture medium and wash cells 1–2 times with 5 mL PBS.
2. Trypsin digestion
- · Add 1 mL of trypsin and gently swirl the flask to ensure the enzyme covers the cell layer evenly.
- · Incubate the flask in a 37 °C incubator for 1–2 minutes.
- · Observe under a microscope: when most cells become rounded, exhibit increased refractivity, and detach easily, immediately stop digestion.
3. Terminate digestion
- · Add 2 volumes (2 mL) of complete medium to neutralize the trypsin.
- · Transfer the cell suspension to a 15 mL centrifuge tube.
4. Centrifugation and resuspension
- · Centrifuge at 1100 rpm for 4 minutes at room temperature.
- · Discard the supernatant and gently resuspend the cell pellet in complete medium.
5. Seeding and culture
- · Seed cells into new flasks at a 1:2 to 1:4 split ratio.
- · Monitor cell growth the following day to ensure successful passaging.
MC38 Cell Cryopreservation Procedure
- 1. Cell collection: Collect cells following the passaging procedure and transfer the trypsinized cells into a centrifuge tube.
- 2. Centrifugation: Centrifuge at 1100 rpm for 4 minutes at room temperature. Carefully discard the supernatant.
- 3. Resuspension and aliquoting
- · Resuspend the cell pellet in cryopreservation medium and adjust the concentration to 1 × 10⁶ cells/mL.
- · Aliquot the cell suspension into cryovials and clearly label each vial with cell line name, passage number, and date.
- 4. Cooling and storage
- · Place the cryovials in a controlled-rate freezing container and store at −80 °C overnight.
- · Transfer the vials to a liquid nitrogen tank the next day for long-term storage.
MC38 Cell Culture Considerations
- 1. Culture medium and serum: Ensure the correct basal medium is used and supplement with an appropriate amount of serum. Store prepared complete medium at 4 °C and use within two weeks.
- 2. Culture environment: Maintain stable temperature, humidity, and CO₂ concentration in the incubator to provide optimal conditions for cell growth.
- 3. Pre-operation preparation: Pre-warm culture medium and trypsin to 37 °C before passaging or thawing to prevent temperature-induced cellular stress.
- 4. Passaging timing:Passage cells when confluence reaches 80–90%, typically every 2–3 days.
- 5. Key points for passaging: Monitor trypsin digestion time and concentration carefully; over- or under-digestion can damage cells. Avoid excessive pipetting to prevent cell membrane damage. If cells are unevenly attached, gently swirl the flask to distribute them evenly.
- 6. Handling non-adherent cells:Check serum quality. Use coated culture vessels if necessary. Consider using 0.05% trypsin with EDTA (e.g., TrypLE™) and shorten digestion time to 30–60 seconds.
- 7. MC38 growth characteristics:MC38 cells grow as a mixture of epithelial-like and rounded cells. Rounded cells attach loosely and require proper trypsin digestion for subculturing.
MC38 Cell Transfection Considerations
1. Cell condition requirements
- · Select cells in the logarithmic growth phase (70–80% confluence) with viability >95% (assessed by Trypan Blue).
- · Use low-passage cells to ensure high transfection efficiency and experimental consistency.
- · Carefully monitor trypsin digestion time to avoid over-digestion and cellular damage.
- · Disperse cells into a single-cell suspension to prevent clumping.
2. Transfection reagents and preliminary experiments
- · Mix transfection reagents thoroughly before use to ensure homogeneity.
- · Perform pilot experiments to determine optimal drug concentrations for subsequent selection.
3. Electroporation
- · Control the number of cells and seed them into appropriately sized culture plates after electroporation.
- · Wash cells 1–2 times with PBS to completely remove serum, minimizing ionic interference.
- · Optimize electroporation parameters in preliminary experiments.
- · Post-electroporation, ensure ≥70% cell attachment.
- · Limit total electroporation time to avoid excessive cellular stress.
4. Lentiviral Transduction
- · Conduct preliminary experiments to determine the optimal MOI.
- · Maintain cell confluence at 30–40% prior to infection; avoid higher densities.
- · Add Polybrene before infection to enhance transduction efficiency.
- · Replace medium 24 hours post-infection to remove residual virus.
- · Avoid repeated freeze-thaw cycles of viral stocks.
5. Lipofection
- · Select a lipofection reagent compatible with MC38 cells and optimize transfection conditions in preliminary experiments.
- · Plate cells 24 hours before transfection to achieve 60–70% confluence at transfection.
- · Dilute DNA in Opti-MEM before adding the lipid reagent (do not mix in reverse).
- · Allow DNA-lipid complexes to incubate at room temperature for 15–20 minutes (<10 min may result in incomplete complex formation; >30 min may increase toxicity).
- · Add complexes slowly and evenly to the culture medium, gently swirling to mix; avoid direct impact or vigorous pipetting of cells.
MC38 Single-Cell Cloning (Clone Screening) Considerations
1. Cell condition
- · Use cells in the logarithmic growth phase. Prior to plating for cloning, maintain cell confluence at approximately 70%.
- · Cell viability should be ≥90% to ensure healthy clone formation after plating.
2. Reagent preparation
- · Pre-warm all reagents (culture medium, trypsin, PBS, etc.) to 37 °C before use.
- · Consider using gentle dissociation reagents, such as TrypLE™ Express, to minimize cellular damage.
3. Plating strategy
- · Conduct preliminary experiments to determine the optimal seeding density for single-cell cloning, avoiding excessively low clone yields.
- · When seeding 96-well plates, ensure even cell distribution. Add PBS to peripheral wells to reduce medium evaporation.
4. Dilution method
- · Use the limiting dilution method for single-cell plating.
- · After counting, the optimal seeding density is 1–2 × 10⁶ cells per plate.
5. Lentiviral infection (optional)
For clone screening following lentiviral transduction, follow appropriate infection protocols to ensure high infection efficiency while maintaining single-clone formation.
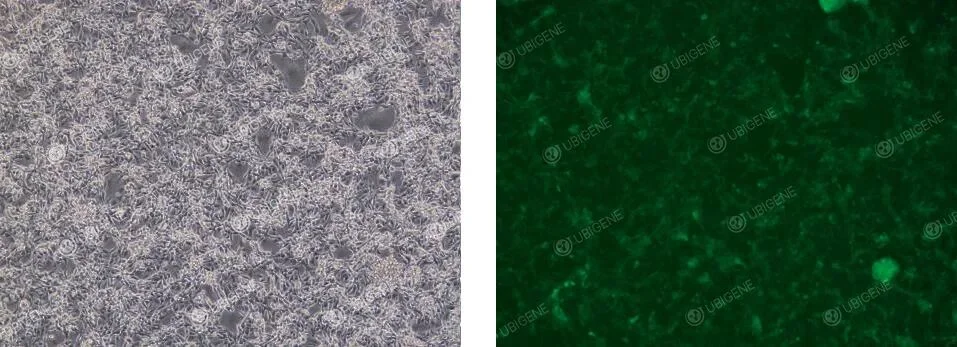
Cell Images of Cells Following Lentiviral Transduction
Common Issues in MC38 Cell Culture and Solutions
1. Cell aggregation and clumping
Observation: Cells do not form a uniform monolayer but grow in clusters of varying sizes.
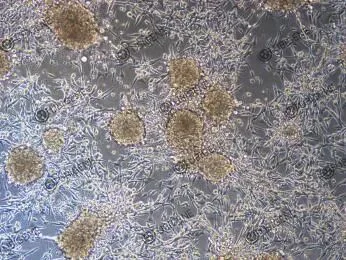
Possible causes:
- · Incomplete digestion: Cells were not fully dissociated into single cells during passaging.
- · Acidic culture medium: High cell density leads to metabolite accumulation and pH decrease, promoting cell aggregation.
Solutions:
- · After digestion, add complete medium and gently pipette up and down multiple times to disperse cell clusters into single cells.
- · Passage cells timely, maintaining density at 50–80%; avoid overconfluence (100% confluence).
2. Poor adhesion or non-adherent cells
Observation: Following digestion and passaging, many cells remain suspended and fail to attach.
Possible causes:
- · Over-digestion: Excessive trypsin exposure damages membrane adhesion proteins.
- · Harsh handling: Vigorous pipetting disrupts cells.
- · Serum issues: Low-quality or insufficient serum fails to provide adequate attachment factors.
Solutions:
- · Limit digestion to 1–2 minutes; monitor under a microscope every minute, and stop digestion when most cells become rounded. Avoid waiting until all cells float.
- · Pipette very gently, allowing liquid to flow along the flask wall; avoid direct impact on the cell layer.
- · Use high-quality FBS at 10% concentration; test new batches before use.
3. Decline in cell condition and morphological changes
Observation: Cells become rounded, cytoplasmic granularity increases, transparency decreases, and cellular debris accumulates.
Possible causes:
- · Medium issues: Incorrect pH (CO₂ imbalance) or depleted nutrients (delayed medium change).
- · Contamination: Bacterial, fungal, or mycoplasma contamination.
Solutions:
- · Maintain CO₂ incubator at 5%; check medium color, and replace if yellowing occurs. For high-density cultures, change medium every 2 days.
- · Observe under a microscope: bacterial contamination usually causes rapid turbidity; fungal contamination presents as filamentous structures; mycoplasma requires specific detection. If microbial contamination is detected, discard affected cultures immediately and perform thorough disinfection.
Quick Tip: The key to successful MC38 cell recovery is gentle handling! Thaw in a water bath, centrifuge to remove the cryoprotectant, plate evenly, and check attachment after 24 hours. Handle with care, and your experiments will run smoothly!
Contact us to get your “dream cells,” and kickstart a highly efficient journey in tumor immunology research.



