Progress in the Cell Stem Cell Journals: Ubigene Empowers Novel Vascular Malformation Models and Accelerates Therapeuti


Progress in the Cell Stem Cell Journals: Ubigene Empowers Novel Vascular Malformation Models and Accelerates Therapeutic Development
Venous malformations (VMs) are the most common type of vascular malformation, primarily caused by somatic mutations in venous endothelial cells (VECs), with an estimated incidence of 1–2 per 20,000 individuals. Clinically, patients typically present with vascular structural abnormalities accompanied by localized pain, swelling, and bleeding. In severe cases, these symptoms can lead to impaired organ function, significantly reducing the patients’ quality of life. Current treatment strategies rely mainly on sclerotherapy and surgical resection, which are constrained by high procedural risk, elevated costs, and limited curative potential. More critically, there are currently no FDA-approved pharmacological therapies for VM. A major barrier lies in the absence of an appropriate disease model. Conventional transgenic animal approaches are unsuitable, as VM mutations are non-heritable and manifest locally. Although researchers have attempted to construct models by delivering genes carrying pathogenic mutations via AAV vectors in mice, such methods are costly, have poor stability, and are limited by human-mouse interspecies differences, making them impractical for widespread use. Therefore, the development of a novel, scalable, and cost-effective disease model that faithfully recapitulates VM pathology is of great significance for advancing mechanistic insights and enabling therapeutic discovery.
Professor Kai Wang and Professor Wei Kong from the National Key Laboratory of Vascular Homeostasis and Remodeling, School of Basic Medical Sciences, Peking University, together with Researcher Qian Wang from the Clinical Stem Cell Research Center, Peking University Third Hospital, and Researcher Zhengwei Xie from the International Cancer Institute, Peking University, have published an important study in Cell Stem Cell. In this work, Ubigene’s THP-1-EGFP human monocytic cell model provided critical technical support that facilitated the progress of the project.
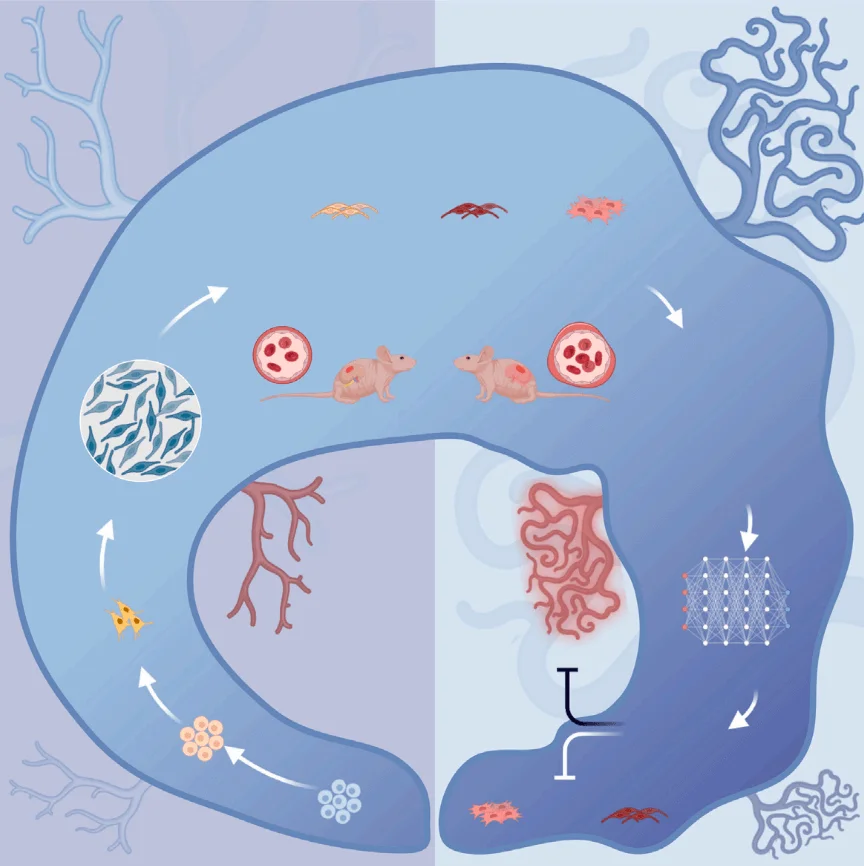
The study, entitled “Generation of iPSC-derived human venous endothelial cells for the modeling of vascular malformations and drug discovery”, reports the development of an efficient protocol for differentiating human induced pluripotent stem cells (iPSCs) into venous endothelial cells (iVECs). Furthermore, by generating iVECs carrying the TIE2-L914F heterozygous mutation, the team successfully recapitulated the pathological features of venous malformations. Leveraging AI-assisted drug prediction and Drug-seq technology, the researchers identified that Bosutinib, an FDA-approved leukemia drug, could effectively ameliorate the vascular malformation phenotype, highlighting its promising clinical potential.
This study focuses on the most prevalent pathogenic mutation in venous malformations (VMs), TIE2 p.L914F (c.2740 C>T).TIE2 (also known as TEK) belongs to the receptor tyrosine kinase family and is predominantly expressed in endothelial cells, where it plays a pivotal role in angiogenesis, vascular remodeling, maturation, and maintenance of vascular integrity.To date, the most commonly used cellular model for vascular malformation research has been HUVECs (human umbilical vein endothelial cells) overexpressing TIE2 mutants. However, such models are subject to significant limitations:
To overcome these challenges posed by the aforementioned HUVEC-based models, the research team established a robust differentiation protocol to generate venous endothelial cells (iVECs) from human induced pluripotent stem cells (iPSCs). By modulating the retinoic acid (RA) signaling pathway to regulate the cell cycle, this protocol promotes the specific differentiation of cells toward the venous endothelial direction. In parallel, researchers employed precise genome editing to introduce the heterozygous L914F mutation into the TIE2 locus of iPSCs, thereby faithfully recreating the patient-specific pathogenic context.
Building upon previous work, the research team systematically optimized differentiation conditions and ultimately established a stable and efficient protocol for generating venous endothelial cells (iVECs). The protocol centers on the use of vascular endothelial growth factor A (VEGFA), fibroblast growth factor 2 (FGF2), the NOTCH inhibitor DAPT, and retinoic acid (RA). Further investigation revealed that RA induces early G1-phase cell cycle arrest, thereby facilitating commitment toward the venous lineage.
Single-cell RNA sequencing comparing iVECs with arterial endothelial cells (iAECs) suggested that the transcription factor MEF2C may promote venous endothelial differentiation—a role not previously reported in arterial–venous specification. Functional validation through MEF2C overexpression confirmed this finding, as the proportion of venous endothelial cells was markedly increased. At the functional level, the team leveraged THP-1-EGFP cells provided by Ubigene to further verify key venous endothelial features of iVECs and iAECs, including:
- · Lower nitric oxide (NO) production
- · Weaker shear stress response
- · Enhanced immune cell recruitment capacity
Moreover, in in vivo transplantation experiments, iVECs not only stably expressed venous markers but also formed perfusable vascular lumens, further demonstrating their robust venous functionality.
Using CRISPR-Cas9 gene editing, the research team precisely introduced the TIE2-L914F heterozygous mutation into iPSCs and subsequently derived iVECs carrying this mutation. The mutant iVECs faithfully recapitulated hallmark pathological features of venous malformations (VMs), including:
- · Cytoskeletal abnormalities— disorganized F-actin architecture
- · Aberrant cellular behavior— enhanced proliferative capacity, increased resistance to apoptosis, and impaired tube formation ability.
Bulk RNA-seq analysis further confirmed the dysregulation of relevant signaling pathways and abnormalities in gene expression levels in mutant cells. When transplanted beneath the renal capsule of mice, the mutant iVECs formed structurally abnormal and functionally defective vessels, thereby validating the pathological authenticity of the model in vivo.
To explore potential therapeutic strategies, the researchers integrated deep learning–based DLEPS drug prediction with Drug-seq profiling, which identified Bosutinib as a promising candidate. Experimental validation demonstrated that Bosutinib treatment markedly alleviated VM-like abnormalities in mutant iVECs. Mechanistic studies further revealed that Bosutinib exerts its therapeutic effects by suppressing endothelial-to-mesenchymal transition (EndMT) and reducing aberrant cellular proliferation, thereby mitigating the pathological manifestations associated with the TIE2-L914F mutation.
In summary, this study elucidates the mechanism by which retinoic acid (RA) promotes venous endothelial specification by inducing G1-phase arrest and activating venous lineage–associated genes. By precisely introducing the TIE2-L914F heterozygous mutation into iPSCs, the researchers successfully established an iVEC model that faithfully recapitulates the pathological features of venous malformations (VMs). Leveraging this platform, the integration of deep learning–based prediction and high-throughput DRUG-seq screening identified Bosutinib, an FDA-approved drug, which markedly ameliorated VM phenotypes both in vitro and in vivo. These findings not only provide a powerful new model for investigating the pathogenesis of VMs but also open new avenues for therapeutic development and clinical intervention in vascular malformation–related diseases.
Support Provided by Ubigene
In this study, THP-1-EGFP cell model provided by Ubigene offered essential technical support that enabled the smooth progression of the project. Currently, Ubigene offers 2,000+ stable cell line products with stable and high-level expression, including widely used tool cell lines such as Luc, EGFP, Cas9, and OVAL cells, as well as stable overexpression lines covering a broad range of popular genes and diverse cell types.These stable cell lines are characterized by low passage numbers, high activity, and excellent growth status. They are constructed either using lentiviral systems or Ubigene’s proprietary EZ-OE™ technology, ensuring robust and stable expression of the target gene. Such models are well-suited to support diverse research needs across multiple fields.
Click here to learn more about Ubigene’s custom stable cell line services>>
Access the Same Stable Cell Lines
Ubigene’s EGFP stable cell lines feature low passage numbers, high viability, and excellent growth status. Constructed via lentiviral transduction, these cell lines stably express the EGFP fluorescent protein, making them ideal for applications such as high-throughput drug screening, in vivo fluorescence tracing, and stable cell line construction.
Browse our cell line bank to explore more EGFP stable cell lines>>
Reference
[1] Behravesh, S., Yakes, W., Gupta, N., Naidu, S., Chong, B. W., Khademhosseini, A., & Oklu, R. (2016). Venous malformations: clinical diagnosis and treatment. Cardiovascular diagnosis and therapy, 6(6), 557–569. https://doi.org/10.21037/cdt.2016.11.10
[2] Uebelhoer, M., Nätynki, M., Kangas, J., Mendola, A., Nguyen, H. L., Soblet, J., Godfraind, C., Boon, L. M., Eklund, L., Limaye, N., & Vikkula, M. (2013). Venous malformation-causative TIE2 mutations mediate an AKT-dependent decrease in PDGFB. Human molecular genetics, 22(17), 3438–3448. https://doi.org/10.1093/hmg/ddt198
[3] Kangas, J., Nätynki, M., & Eklund, L. (2018). Development of Molecular Therapies for Venous Malformations. Basic & clinical pharmacology & toxicology, 123 Suppl 5, 6–19. https://doi.org/10.1111/bcpt.13027
[4] Cooke-Barber, J., Kreimer, S., Patel, M., Dasgupta, R., & Jeng, M. (2020). Venous malformations. Seminars in pediatric surgery, 29(5), 150976. https://doi.org/10.1016/j.sempedsurg.2020.150976



