Progress in the Nature Series Journals: Ubigene Advances the Discovery of Novel Anti-Cancer Targets


Progress in NatureSeriesJournals: Ubigene Advances the Discovery of Novel Anti-Cancer Targets — In Vivo and In Vitro Evidence Demonstrates Significant Suppression of Tumor Malignancy
Doublecortin-like kinase 1 (DCLK1), an emerging marker of cancer stem cells, is activated during tumorigenesis, endowing cells with stem-like properties and promoting metastasis. However, the molecular mechanisms underlying its activation in tumors — and the rationale for selectively adopting specific alternative promoter (AP) usage patterns — remain largely unclear.
On August 7, a research team from Capital Medical University published an important study in Cell Death & Disease, a sub-journal of Nature. In this work, Ubigene provided critical vector models that served as essential technical support, enabling the successful progression of the project. The article, entitled “Hypoxic stimulation of DCLK1 transcription and alternative-promoter switching fuels tumor malignancy in clear cell renal cell carcinoma,” reports novel mechanistic insights into tumor biology.
This study is the first to demonstrate that in clear cell renal cell carcinoma (ccRCC), DCLK1 is markedly activated in conjunction with a switch in promoter usage, favoring expression of the long isoform (DCLK1-L). Mechanistically, this process is driven by the hypoxia–HIF2α–PLOD2 axis, which in turn activates β-catenin, promoting its selective binding and activation of the α-promoter. The findings reveal that hyperactivation of the PLOD2–DCLK1-L axis is closely associated with elevated epithelial–mesenchymal transition (EMT) signatures and correlates with poor prognosis in ccRCC patients. Notably, pharmacological targeting of DCLK1-L to disrupt this signaling pathway significantly attenuated malignant tumor progression in both in vitro and in vivo models.

Background
Renal cell carcinoma (RCC) is one of the most common malignant tumors of the urinary system in adults. According to global cancer epidemiology statistics, RCC accounted for 179,368 deaths in 2020, the majority of which were due to distant metastases. Clear cell renal cell carcinoma (ccRCC) represents the predominant histological subtype, comprising 70–90% of all RCC cases and approximately 83–88% of metastatic RCC. The vast majority of ccRCC cases are closely associated with the inactivation of the VHL tumor suppressor gene and the resulting dysregulation of hypoxia signaling pathways.
Targeting DCLK1 Mitigates the Malignant Progression of Hypoxia- and PLOD2-Enriched Clear Cell Renal Cell Carcinoma In Vitro and In Vivo
To evaluate the potential clinical value of inhibiting this signaling axis, the researchers identified DCLK1 as a key druggable molecule and employed the selective inhibitor DCLK1-IN-1, which effectively suppresses both DCLK1 expression and its phosphorylation. Results showed that under hypoxic conditions or PLOD2 overexpression, DCLK1-IN-1 significantly reduced the induction of DCLK1-L, thereby effectively blocking hypoxia- and PLOD2-driven epithelial–mesenchymal transition (EMT), migration, invasion, and acquisition of stem-like properties in vitro.
Furthermore, systemic administration of DCLK1-IN-1 confirmed its therapeutic efficacy in PLOD2-enriched ccRCC xenograft models in vivo. Specifically, ccRCC cells were subcutaneously inoculated into NOD/SCID mice to establish PLOD2-rich xenografts, which were subsequently retransplanted into BALB/c nude mice. Tumor-bearing mice were randomized into a DMSO control group and a DCLK1-IN-1 treatment group (n = 8 per group). Following oral gavage every other day for 16 days (25 mg/kg), the DCLK1-IN-1 group exhibited markedly reduced tumor volume, weight, and growth rate, achieving an inhibition rate of 85.1%.
In addition, RT-qPCR and IHC analyses demonstrated that DCLK1-IN-1 treatment significantly suppressed EMT phenotypes and stem-like features in PLOD2-enriched ccRCC xenografts. This was evidenced by increased expression of the epithelial marker E-cadherin, decreased expression of the mesenchymal marker vimentin, and reduced expression levels of the cancer stem cell marker Nanog.
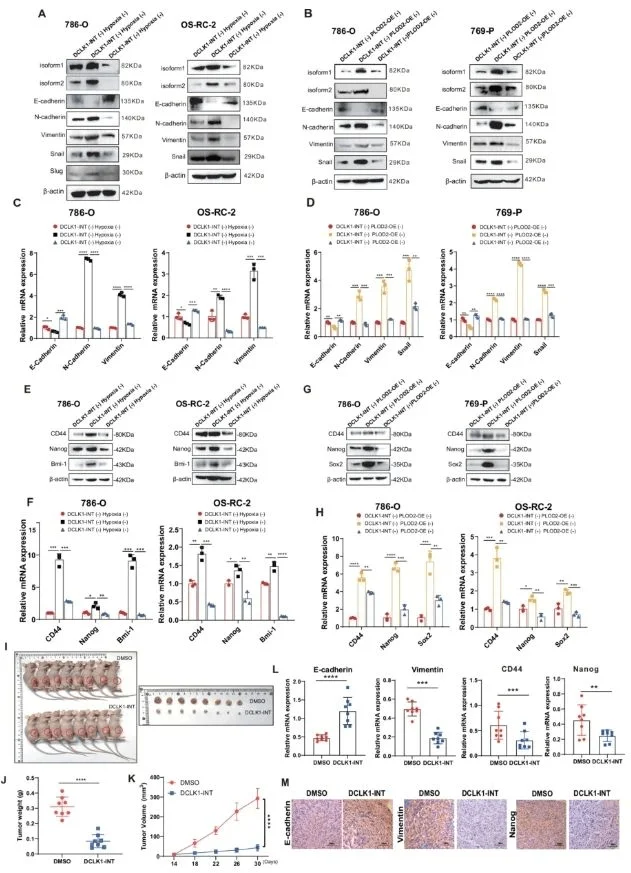
Targeting DCLK1 attenuates the malignant progression of hypoxia- and PLOD2-enriched clear cell renal cell carcinoma both in vitro and in vivo.
In summary, these findings suggest that ccRCC patients with hypoxic and PLOD2-high tumors may benefit from DCLK1-targeted inhibition, highlighting the potential clinical therapeutic value of DCLK1 in this patient subgroup.
Support Provided by Ubigene
Throughout this study, Ubigene provided crucial technical support for experimental design and implementation. With extensive expertise in gene editing technologies, Ubigene delivers customized solutions tailored to diverse research needs, encompassing the full workflow from vector construction and cell model generation to in vitro and in vivo CRISPR library screening and subsequent functional validation.
Contact Ubigene to learn more about our specialized gene editing solutions and comprehensive research support!



