Ubigene Drives Advances in Liver Cancer Research | miR-122-5p Modulates hiPSCs to Suppress EMT


Ubigene Drives Advances in Liver Cancer Research | miR-122-5p Modulates hiPSCs to Suppress EMT, Highlighting Potential for Cell-Based Therapies
Liver cancer ranks among the five most prevalent and high-risk malignancies worldwide and remains the only cancer type with a consistently rising incidence. China alone bears nearly half of the global disease burden. Owing to its insidious onset and lack of early clinical symptoms, most patients are diagnosed at advanced stages, leading to poor prognosis and markedly reduced quality of life. These challenges underscore the urgent need to identify novel therapeutic targets and effective treatment strategies.
On May 6, a research team from Tianjin Third Central Hospital published important findings in the European Journal of Histochemistry. In this study, Ubigene provided key cell tools, enabling the successful progression of the project. The article, entitled “MiR-122-5p inhibits the epithelial–mesenchymal transition of liver cancer cells by inducing hiPSCs to differentiate into hepatocyte-like cells”, investigated the role of miR-122-5p in directing human induced pluripotent stem cells (hiPSCs) toward hepatocyte-like differentiation and its impact on the epithelial–mesenchymal transition (EMT) of liver cancer cells.The results demonstrated that:
- - Overexpression of miR-122-5p in hiPSCs significantly increased the expression of liver-specific markers (AFP, ALB, ASGPR), thereby promoting differentiation into hepatocyte-like cells, while knockdown of miR-122-5p produced the opposite effect.
- - In a co-culture system with HepG2 liver cancer cells, hiPSCs overexpressing miR-122-5p suppressed EMT, evidenced by decreased mesenchymal markers and increased epithelial markers.
- - Moreover, these modified hiPSCs inhibited HepG2 cell proliferation, invasion, and migration, while simultaneously inducing apoptosis.
Collectively, the findings suggest that miR-122-5p not only facilitates the differentiation of hiPSCs into hepatocyte-like cells but also inhibits EMT in liver cancer cells, highlighting miR-122-5p as a promising therapeutic target for the development of cell-based strategies to prevent liver cancer progression.

Background
Liver cancer is among the five most prevalent high-risk cancers worldwide and the only type with a steadily increasing incidence. Epithelial–mesenchymal transition (EMT) is a key process driving tumor metastasis, during which epithelial cells undergo structural alterations—losing polarity and cell–cell junctions—while acquiring mesenchymal-like morphology and functional traits. This transition markedly enhances the proliferative, migratory, and invasive capacities of cancer cells, placing EMT at the core of liver cancer progression and metastasis, and making it an important therapeutic target.
Current hepatocyte-based therapies face significant limitations: liver transplantation remains a major treatment option but carries high mortality rates; hepatocyte transplantation (HCTx) has shown promise as an alternative but is constrained by donor scarcity and limited regenerative capacity of transplanted cells. In contrast, human induced pluripotent stem cells (hiPSCs) offer high proliferative potential, low immunogenicity, and multipotency. They can be infinitely expanded and differentiated into diverse cell types while avoiding the ethical and scientific concerns of embryonic stem cells, providing new possibilities for liver cancer cell therapy.
MicroRNAs (miRNAs) are a class of 20–23 nucleotide non-coding RNAs that regulate gene expression by binding to the 3′-UTR of target genes, and they have emerged as feasible therapeutic targets in cancer. Previous studies have indicated that miR-122-5p may function as a tumor suppressor in liver cancer, for example by predicting hepatocellular carcinoma (HCC) prognosis and inhibiting the proliferation and migration of intrahepatic cholangiocarcinoma (ICC) and nasopharyngeal carcinoma. However, its precise role in liver cancer therapy—particularly its involvement in hiPSC differentiation and regulation of EMT in liver cancer cells—remains unclear. This study therefore focuses on elucidating the regulatory effects of miR-122-5p on hiPSC differentiation and EMT in liver cancer cells.
miR-122-5p Promotes Differentiation of hiPSCs into Hepatocyte-like Cells and Suppresses EMT in Liver Cancer Cells
In this study, human induced pluripotent stem cells (hiPSCs) provided by Ubigene were cultured under feeder-free conditions using the EZ-Stem™ complete stem cell medium. During culture, the hiPSCs spontaneously formed well-defined, compact colonies with clear boundaries, and healthy colonies exhibited smooth fusion and robust in vitro growth stability. Importantly, immunofluorescence staining confirmed strong expression of core pluripotency markers, including transcription factors OCT4 and SOX2, as well as the nuclear protein NANOG (Figure 1B). These results validated the pluripotent properties of the hiPSCs and established a solid foundation for subsequent investigations into the role of miR-122-5p in differentiation and EMT regulation.

Figure.1
miR-122-5p Facilitates Differentiation of hiPSCs into Hepatocyte-like Cells
To evaluate the role of miR-122-5p in hiPSC differentiation, researchers constructed overexpression and knockdown plasmids and transfected them into hiPSCs, followed by assessment of cell proliferation and hepatocyte marker expression. The results demonstrated that:
- - Cell proliferation: CCK8 assays revealed that miR-122-5p overexpression significantly enhanced the proliferative capacity of hiPSCs, whereas knockdown markedly reduced proliferation.
- - Hepatocyte markers: Flow cytometry showed that miR-122-5p upregulation increased the positive expression rate of the hepatocyte-specific surface receptor ASGPR, while knockdown produced the opposite effect. Immunofluorescence confirmed that hiPSCs with miR-122-5p overexpression exhibited elevated expression of AFP, ALB, and ASGPR.
- - Gene expression: RT-qPCR analysis indicated that overexpression of miR-122-5p significantly upregulated hepatocyte-specific genes, including ALB, HNF4α, and CK18.
Together, these findings suggest that miR-122-5p overexpression promotes the differentiation of hiPSCs into hepatocyte-like cells.
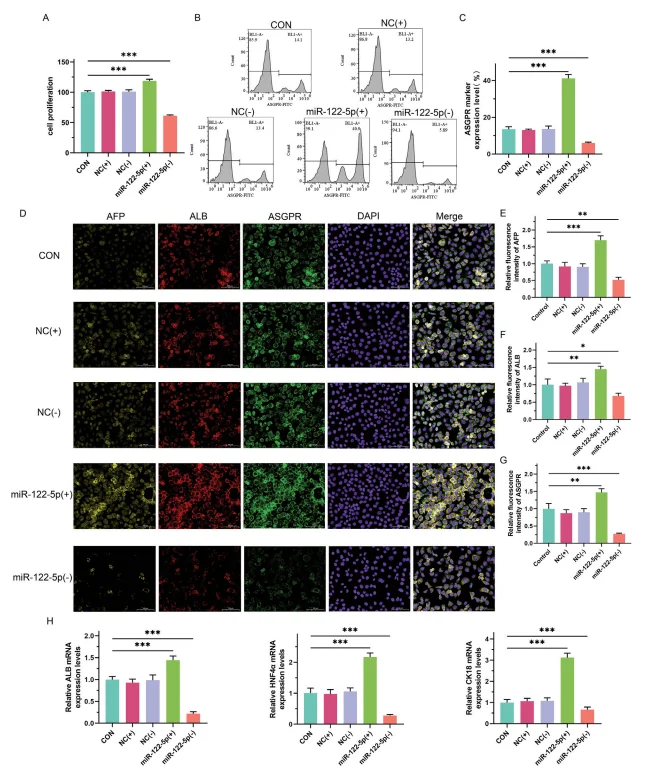
Figure. 2
miR-122-5p-Modified hiPSCs Suppress Malignant Phenotypes and EMT in HepG2 Cells
To further investigate the functional effects of miR-122-5p, the researchers examined the impact of miR-122-5p-modified hiPSCs on the malignant phenotype and EMT of HepG2 liver cancer cells in a co-culture system:
- - Cell proliferation: CCK8 assays demonstrated that hiPSCs overexpressing miR-122-5p significantly inhibited HepG2 proliferation, whereas miR-122-5p knockdown promoted cell growth.
- - Migration and invasion: Transwell and wound-healing assays revealed that co-culture with miR-122-5p-overexpressing hiPSCs markedly reduced the migratory and invasive capacities of HepG2 cells.
- - Apoptosis: Flow cytometry analysis showed that hiPSCs overexpressing miR-122-5p induced higher apoptosis rates in HepG2 cells.
Collectively, these findings suggest that miR-122-5p not only promotes the differentiation of hiPSCs into hepatocyte-like cells but also suppresses the malignant progression of liver cancer cells by inhibiting proliferation, migration, and invasion, while inducing apoptosis.
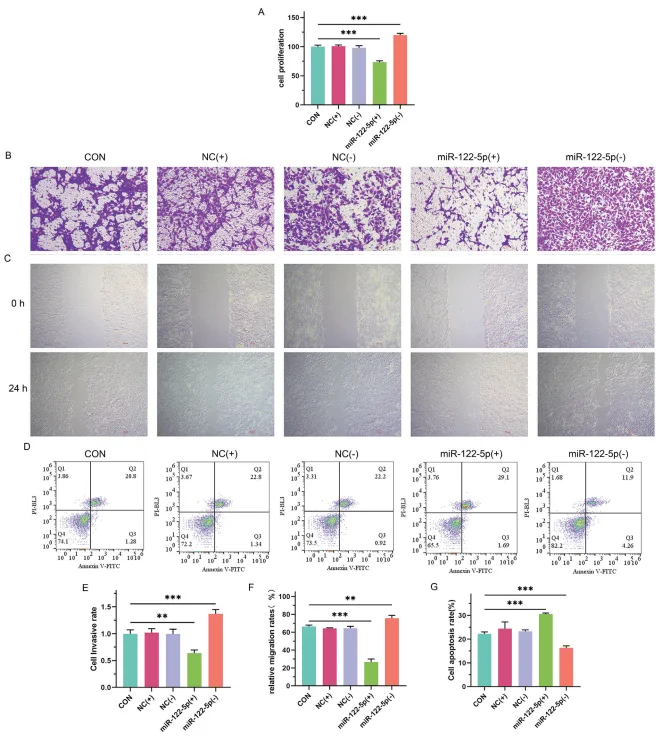
Figure. 3
miR-122-5p Regulates EMT Marker Expression to Suppress EMT in HepG2 Cells
To further investigate the role of miR-122-5p in EMT regulation, RT-PCR, Western blot, and immunofluorescence assays were performed to assess the expression of EMT-related markers at both gene and protein levels.
- - Gene expression: RT-PCR analysis showed that HepG2 cells co-cultured with hiPSCs overexpressing miR-122-5p exhibited significantly increased mRNA levels of the epithelial marker E-Cadherin and decreased levels of the mesenchymal marker N-Cadherin. Conversely, co-culture with miR-122-5p knockdown hiPSCs resulted in E-Cadherin downregulation and N-Cadherin upregulation.
- - Protein expression: Western blot assays confirmed these trends, with E-Cadherin protein expression increased and N-Cadherin decreased in HepG2 cells co-cultured with miR-122-5p-overexpressing hiPSCs.
- - Immunofluorescence: Consistent with these findings, immunofluorescence staining demonstrated enhanced E-Cadherin signal intensity and reduced N-Cadherin signal intensity in HepG2 cells.
Collectively, these results suggest that miR-122-5p promotes the differentiation of hiPSCs into hepatocyte-like cells and suppresses the EMT process in liver cancer cells, which may represent a key mechanism underlying its inhibitory effect on liver cancer progression.
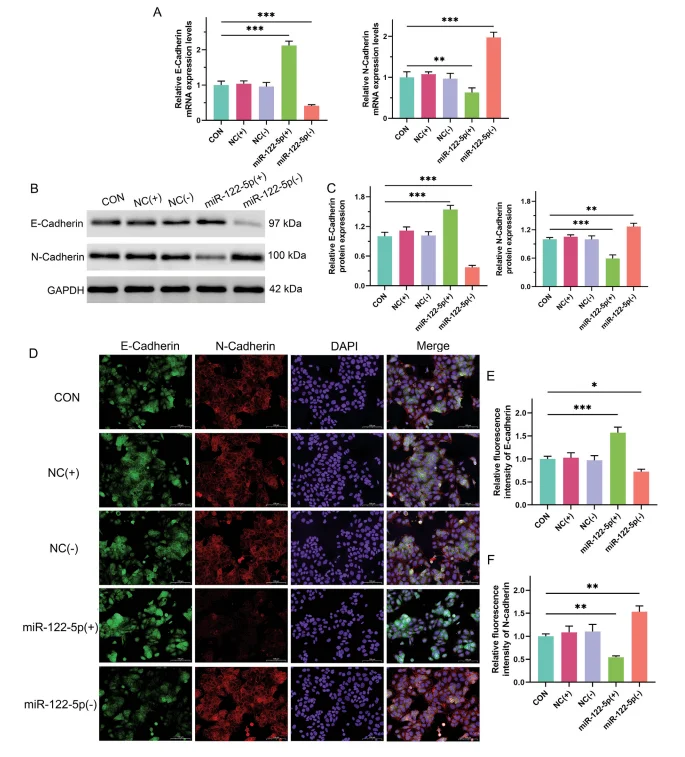
Figure. 4
Support Provided by Ubigene
In this study, the Human induced pluripotent stem cells (iPSC) and EZ-Stem™ complete stem cell medium were provided by Ubigene, which played a critical role in enabling the research team to validate the key mechanism of “miR-122-5p regulating hiPSC differentiation to suppress EMT in liver cancer cells.”Ubigene maintains a comprehensive ready-to-use cell line bank, encompassing 1,000+ wild-type cell lines, 8,000+ knockout cell lines, and 2,000+ Tool cell lines (e.g., Luc, Cas9, EGFP). These resources allow research teams to rapidly access the same cell lines used in this study or to initiate related experiments, thereby accelerating scientific discovery and translational research.
Quickly browse our cell line bank to access your target cell lines >>
Reference
Xing Q, Xu Y, Luo Y, Li C, Wang P, Kang B, Lu C. MiR-122-5p inhibits the epithelial mesenchymal transition of liver cancer cells by inducing hiPSCs to differentiate into hepatocyte-like cells. Eur J Histochem. 2025 Apr 7;69(2):4190. doi: 10.4081/ejh.2025.4190. Epub 2025 May 6. PMID: 40336362; PMCID: PMC12086357.



