STTT | Ubigene’s Knockout Cell Models Facilitate the Discovery of NINJ1’s Critical Role in Influenza-Induced PANoptos


STTT | Ubigene’s Knockout Cell Models Facilitate the Discovery of NINJ1’s Critical Role in Influenza-Induced PANoptosis
In September 2025, Professor Bin Cao’s team at China-Japan Friendship Hospital published their latest findings in Signal Transduction and Targeted Therapy, systematically elucidating the critical role of the transmembrane protein Ninjurin-1 (NINJ1) during influenza A virus (IAV) infection. Ubigene provided essential support for this study by supplying NINJ1 knockout cell lines (A549 and THP-1), which were instrumental in uncovering NINJ1’s function in mediating PANoptosis.
The study revealed that NINJ1 does not govern the initiation or progression of IAV-induced PANoptosis (programmed necrotic cell death complex). However, it serves as a critical executioner for terminal plasma membrane rupture and the release of damage-associated molecular patterns (DAMPs). Activation of NINJ1 promotes the release of inflammatory mediators and partially regulates IL-1β secretion, thereby amplifying host inflammatory responses.
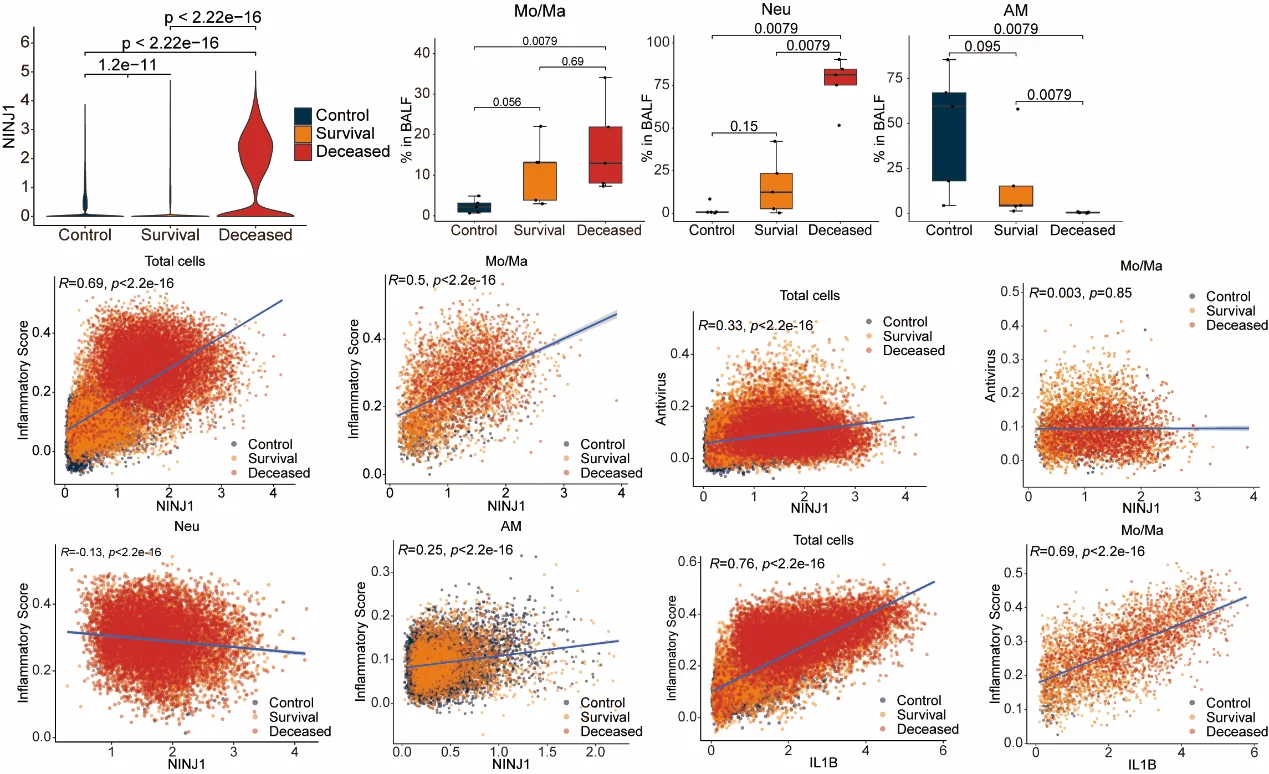
In mouse models, NINJ1 deficiency markedly alleviated lung immunopathology caused by influenza virus infection and improved survival rates. Single-cell transcriptomic analysis further demonstrated that high NINJ1 expression in monocytes/macrophages correlates with disease severity and poor prognosis in COVID-19 patients.This study not only elucidates the mechanistic role of NINJ1 as a downstream effector in viral infection but also highlights its potential as a therapeutic target and biomarker for viral pneumonia. These findings provide new insights into virus-induced PANoptosis and inflammatory responses and lay a foundation for the development of future clinical intervention strategies.
Background
Severe pneumonia caused by influenza A virus (IAV) infection is largely driven by host immune-mediated tissue damage resulting from excessive activation of inflammatory cell death pathways. The recently proposed concept of PANoptosis refers to the integrated activation of pyroptosis, apoptosis, and necroptosis, which often leads to extensive cell lysis and the release of intracellular contents, thereby triggering a strong inflammatory response and exacerbating tissue injury.
Current intervention strategies primarily target upstream regulators of PANoptosis (e.g., ZBP1) or key execution proteins within specific pathways (e.g., GSDMD, GSDME, MLKL). However, animal studies have shown inconsistent protective effects: blocking a single pathway may fail due to functional redundancy among multiple death pathways, while over-inhibition of critical molecules can impair essential antiviral immunity, producing adverse effects.
NINJ1 (Ninjurin-1), a transmembrane protein identified in recent years, has been shown to mediate plasma membrane rupture across various cell death modalities, serving as a key executioner of terminal cell lysis. Nevertheless, its precise role and mechanism in IAV-induced PANoptosis—a complex pathological process—remain largely unexplored.
Research Results
1.IAV infection upregulates NINJ1 expression, correlating with disease severity
The study first confirmed that IAV infection significantly upregulates NINJ1 expression. Specifically, NINJ1 levels were markedly elevated in mouse lung tissue and bone marrow-derived macrophages (BMDMs). Single-cell transcriptomic analysis further revealed that this upregulation was predominantly observed in myeloid cell populations, including macrophages and neutrophils.
Notably, under lethal-dose infection conditions, NINJ1 expression was substantially higher than that observed under sublethal doses, suggesting a positive correlation between NINJ1 expression levels and disease severity.

2. NINJ1 oligomerization is synchronized with PANoptosis and mediates cell lysis
In IAV-infected bone marrow-derived macrophages (BMDMs), the study found that NINJ1 oligomerization—an activation marker—occurs in close temporal synchrony with PANoptosis-associated molecular events, including GSDMD cleavage, MLKL phosphorylation, and Caspase activation. These findings indicate that NINJ1 activation is tightly coupled with the progression of PANoptosis.
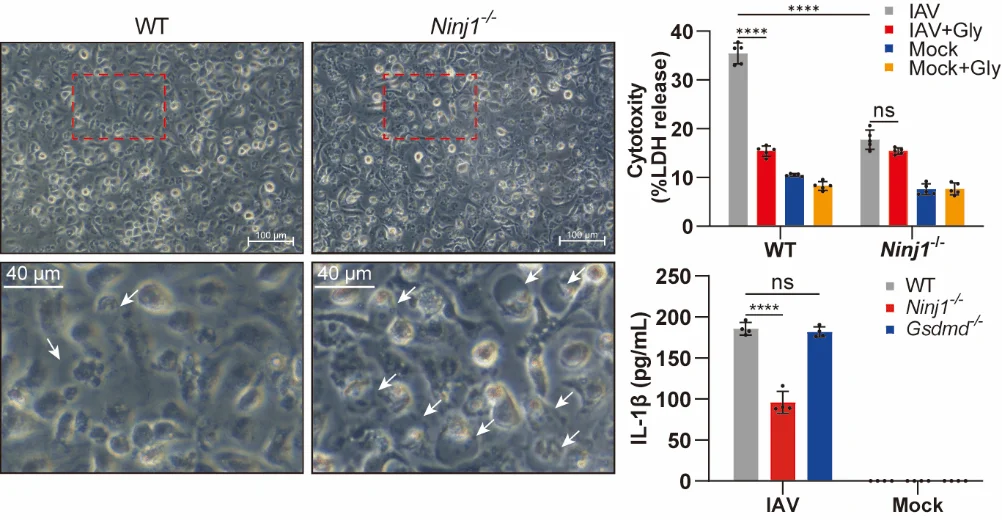
Using Ninj1⁻/⁻ cells, the research team further demonstrated that NINJ1 is essential for IAV-induced plasma membrane rupture and the release of DAMPs such as LDH and HMGB1. Notably, NINJ1 deficiency did not affect the overall rate of cell death, suggesting that its primary function lies in executing the terminal membrane rupture step rather than initiating programmed cell death.
A key finding is that, within the context of IAV-induced PANoptosis, NINJ1 deficiency significantly reduced IL-1β release, whereas GSDMD knockout did not produce a similar effect. This indicates that cytokine release during PANoptosis exhibits pathway specificity, differing from the classical pyroptosis model where IL-1β release is predominantly GSDMD-dependent.
3. NINJ1 activation depends on ZBP1 and can be triggered by any PANoptosis pathway
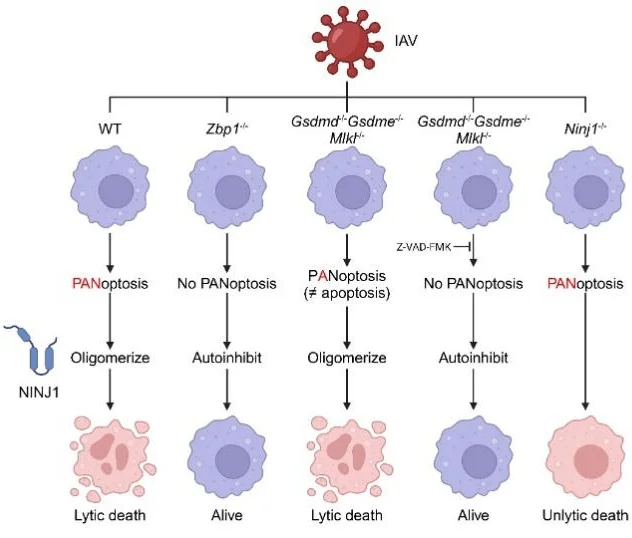
Previous studies have shown that ZBP1 senses viral Z-RNA to initiate cell death during influenza infection. This study further confirmed that NINJ1 oligomerization and the resulting plasma membrane rupture are entirely dependent on ZBP1.
Mechanistic investigations revealed that even when Gsdmd, Gsdme, and Mlkl were simultaneously knocked out, thereby blocking pyroptosis and necroptosis pathways, the remaining apoptotic pathway was still sufficient to activate NINJ1 and trigger cell lysis. Only when apoptosis was additionally inhibited using caspase inhibitors was NINJ1 oligomerization and activation completely abrogated.
These results indicate that the three canonical cell death pathways in PANoptosis exhibit high redundancy in activating NINJ1: activation of any single pathway is sufficient to drive terminal plasma membrane rupture. Consequently, in the context of PANoptosis, apoptotic activation does not correspond to classical “non-inflammatory” cell death but can be converted via NINJ1 into an inflammatory, lytic form of cell death.

4. In vivo validation: NINJ1 deficiency confers a unique protective phenotype
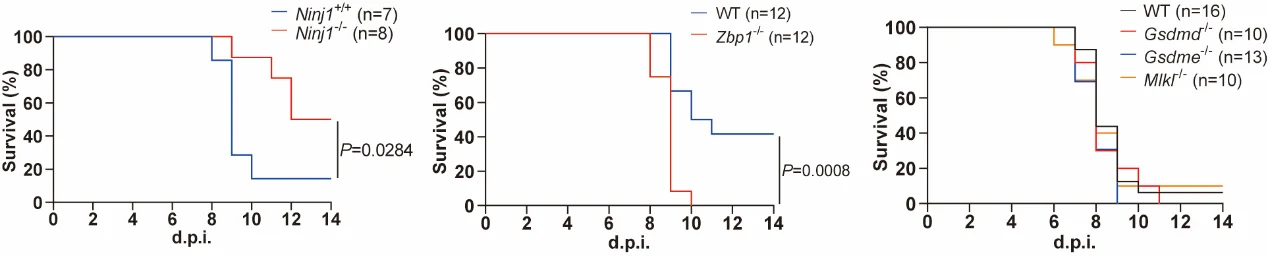
In vivo experiments demonstrated that Ninj1⁻/⁻ mice exhibited significantly improved survival and attenuated lung pathology following lethal-dose IAV infection. Analysis of bronchoalveolar lavage fluid (BALF) revealed markedly reduced protein leakage and lower levels of key inflammatory cytokines (IL-1β, IL-6, TNF-α), while pulmonary viral loads remained unaffected.
Interestingly, contrary to previous reports, Gsdmd⁻/⁻, Gsdme⁻/⁻, and Mlkl⁻/⁻ mice did not show any survival advantage under the experimental conditions of this study. These findings suggest that in the integrated PANoptosis pathway, inhibition of a single branch may fail due to compensation by other pathways, whereas targeting NINJ1, a common downstream executioner of multiple pathways, provides more effective in vivo protection.
Comparison with Zbp1⁻/⁻ mice further highlights the advantages of NINJ1 targeting: although Zbp1⁻/⁻ mice survived infection, their lung viral loads were significantly higher, indicating impaired antiviral immunity. In contrast, Ninj1⁻/⁻ mice not only showed reduced inflammatory responses but also maintained normal viral clearance, demonstrating a more favorable protective phenotype.
5. Clinical relevance: NINJ1 expression correlates with COVID-19 severity
Analysis of single-cell transcriptomic data from bronchoalveolar lavage fluid (BALF) of severe COVID-19 patients revealed that NINJ1 expression in monocytes/macrophages positively correlates with disease severity and inflammatory responses.
These findings suggest that the role of NINJ1 is not limited to influenza infection but may represent a key mechanism common to severe viral pneumonia. The consistent association of NINJ1 with multiple virus-induced inflammatory pathologies further strengthens its potential as a therapeutic target and clinical biomarker.
Research Significance and Outlook
This study systematically elucidates the critical role of NINJ1 in IAV-induced PANoptosis and provides new directions for the treatment of severe viral pneumonia:
- 1. Clarifying the functional mechanism: NINJ1 acts as a key executioner mediating terminal cell lysis in PANoptosis, with activation that exhibits pathway redundancy, integrating multiple programmed cell death signals.
- 2. Revealing unique phenomena: Within the PANoptosis context, activation of a single apoptotic pathway is sufficient to trigger inflammatory cell lysis via NINJ1. In addition, NINJ1 partially regulates IL-1β release independent of GSDMD, enriching our understanding of inflammatory cell death regulation.
- 3. Identifying a potential therapeutic target: Compared with targeting upstream regulators or specific execution proteins, intervening on NINJ1 can effectively alleviate immunopathology while preserving antiviral immune functions, demonstrating superior therapeutic potential.
- 4. Providing clinical insights: NINJ1 expression in myeloid cells correlates closely with disease severity, highlighting its potential as a prognostic biomarker for viral pneumonia.
These findings not only deepen mechanistic understanding of PANoptosis and virus-induced inflammation but also lay a foundation for future clinical intervention strategies.
Looking ahead, future research could focus on the following directions:
- 1. Develop specific NINJ1-targeting inhibitors(e.g., neutralizing antibodies or small molecules) to investigate their therapeutic potential in virus-induced inflammatory responses.
- 2. Validate the role and therapeutic value of NINJ1in broader models of viral pneumonia, including SARS-CoV-2 infection.
- 3. Elucidate cell-type-specific functions of NINJ1, such as in neutrophils, to expand systematic understanding of its immunological roles.
This study not only provides a new perspective on the molecular execution mechanisms of PANoptosis but also opens avenues for novel clinical strategies in the prevention and treatment of severe viral pneumonia.
Support Provided by Ubigene
In the study by Professor Bin Cao’s team, published in Signal Transduction and Targeted Therapy, Ubigene provided NINJ1 knockout cell lines (A549 and THP-1), which played a critical role in elucidating the mechanism by which NINJ1 mediates cell lysis and inflammatory responses during IAV-induced PANoptosis.
Currently, Ubigene offers a KO Cell Line Bank of over 8,000 knockout cell lines, with prices starting from $990. If the desired knockout model is not available in the cell line bank, customized knockout cell line generation services are also offered. Leveraging the proprietary CRISPR-U™ technology, Ubigene achieves a knockout efficiency of up to 97%, surpassing traditional methods and providing highly efficient and reliable technical support for your research projects.
Contact us for more technical support and inquiries>>
Reference
Xu, Y., Zheng, Y., Liu, Y. et al. Ninjurin-1 mediates cell lysis and detrimental inflammation of PANoptosis during influenza A virus infection. Sig Transduct Target Ther 10, 307 (2025). https://doi.org/10.1038/s41392-025-02391-9



