Insights | Tips for U-2OS Cell Culture and Gene Editing


Insights | Tips for U-2 OS Cell Culture and Gene Editing

U-2OS cells, a shining star cell line in the field of osteosarcoma research, have become an indispensable and powerful tool for cancer research and drug screening. Derived from human osteosarcoma, these cells not only perfectly match the complex nature of tumor biology but also provide an ideal experimental platform for in-depth analysis of genetic mutations. Additionally, U-2OS cells offer vast exploratory opportunities for studying drug responses. Today, I will guide you through this mysterious domain of cells and share some tips on U-2OS cell culture and gene editing to help you advance further in your research journey!
U-2 OS Cell Information
Cell Name: U-2OS (Human Osteosarcoma Cell)
Cell Morphology: Epithelial or polygonal, adherent cells.
Culture Conditions: 90% McCoy's 5A + 10% FBS
Gas Phase: Air 95% and Carbon Dioxide 5%
Temperature: 37℃
Media Change Frequency: Every 2-3 days
Passage Ratio: 1:3 to 1:5
Normal Cell Growth Image: Cells typically appear epithelial or polygonal, with clear boundaries, fairly regular shapes. Healthy U-2OS cells exhibit a typical cobblestone or pebble-like arrangement, with cells closely packed but not overlapping, smooth edges, and no prominent protrusions or shrinkage.
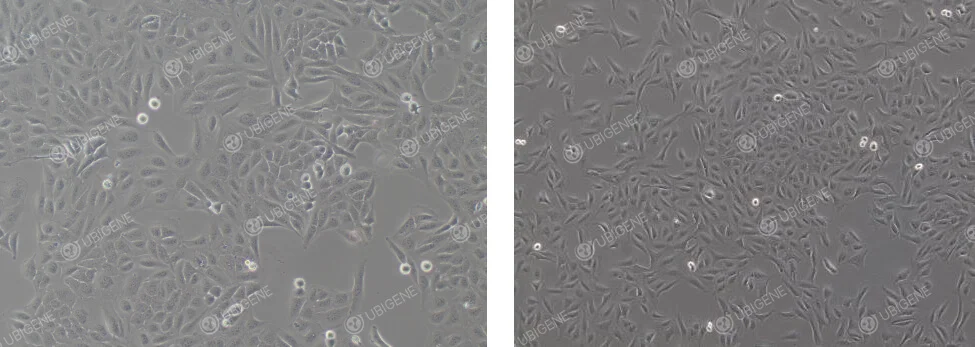
Poor Cell Growth Image: Cell morphology changes, with cells becoming rounded. The cytoplasm may vacuolate, and cells may collapse, losing their three-dimensional structure. Signs of aging are present.
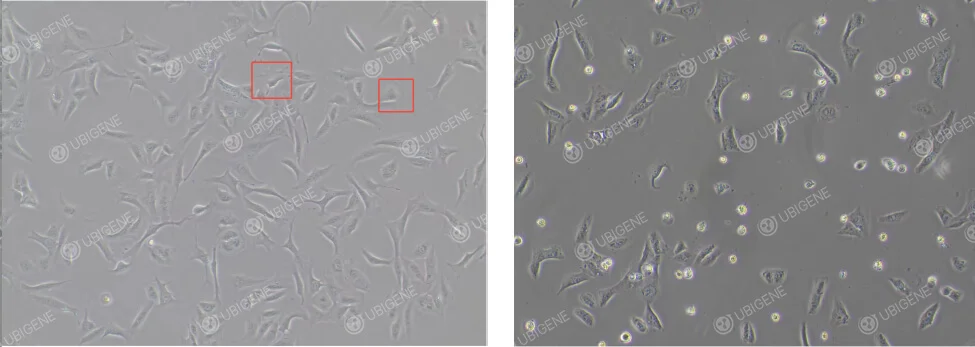
U-2OS cells are commonly used in gene editing experiments, employing transfection methods such as lipid-mediated transfection or electroporation. They can be used for gene knockout (KO), gene knock-in (KI), and point mutation experiments. Their ease of culture, high genomic stability, and transfection efficiency make them an ideal model for functional research.
Cell Revival:
1) Preparation: Prepare 7 mL of complete culture medium in a centrifuge tube for later use.
2) Thawing: Quickly remove the cells from dry ice, hold the lid with tweezers, and place them into a 37°C water bath. Gently agitate until fully thawed (note: water should not cover the lid). This typically takes about 1 minute, stopping when the ice has melted to the size of a green bean.
3) Centrifugation: Transfer the thawed cell suspension to a centrifuge tube and centrifuge at 1100 rpm for 4 minutes. Discard the supernatant.
4) Resuspension and Seeding: Resuspend the cells in complete culture medium and seed them into appropriately sized culture dishes or flasks.
5) Culturing: Place the culture dishes or flasks in a 37℃ incubator and observe cell status and adherence after 24 hours.
Cell Passaging (for T25 flasks as an example):
1) When cells reach 80-90% confluency, passage them by discarding the culture medium in a sterile hood and washing the cells with 5 mL PBS 1-2 times.
2) Add 1 mL trypsin, gently swirl the flask to ensure trypsin covers the cells, then incubate in the incubator for 1-2 minutes. Monitor under a microscope; when most cells appear rounded and bright, and upon gentle agitation, most cells detach, immediately stop digestion.
3) Add 2 mL of complete culture medium (double the trypsin volume) to stop digestion, then transfer the mixture to a 15 mL centrifuge tube.
4) Centrifuge at 1100 rpm for 4 minutes at room temperature. Discard the supernatant, and resuspend the cells in complete culture medium.
5) Passage the cells at a ratio of 1:3 to 1:5 and observe cell status the following day.
Cell Freezing:
1) Collect Cells: Follow the cell passaging process to collect the digested cells into a centrifuge tube.
2) Centrifugation: Centrifuge at 1100 rpm for 4 minutes and discard the supernatant.
3) Resuspension and Freezing: Resuspend the cells in cryopreservation solution, distributing them into cryogenic tubes at at a density of 1×10^6 cells/mL, and label with name, passage number, and date.
4) Cooling and Storage: Place the cryogenic tubes in a programmed cooling box, store them overnight at -80℃, then transfer them to a liquid nitrogen tank for preservation.
Adjustments for Poor Cell State:
1. Culture Medium and Serum: Ensure the correct basal medium is used and adjust the serum concentration appropriately based on cell condition.
2. Culture Environment: Check if the cultivation temperature, humidity, and gas phase conditions are normal.
3. Avoid using expired or long-stored media: Freshly prepared complete media should be used within two weeks.
4. Cell Passaging Procedures: During passage, monitor digestion time and trypsin concentration to avoid cell damage caused by prolonged or insufficient digestion.
5. Investigate whether over-digestion or abnormal pH values of culture medium could be affecting the cells, adjust digestion time and pH accordingly.
Adjustments for Cellular Aging:
1. Confirm that the cultivation conditions and environment are correct.
2. Avoid Over-Digestion: Use an appropriate trypsin concentration and digestion time; avoid forceful pipetting to prevent cell damage.
3. Increase Serum Concentration: Choose high-quality fetal bovine serum to support cell health.
4. Adjust Cell Density: High cell density may lead to nutrient deprivation and waste accumulation, accelerating cellular aging; maintain cell density within suitable ranges.
5. Add Growth Factors: Can promote cell proliferation and help ameliorate aging states.
6. Change to Low-Passage Cells: Use low-passage cells to ensure optimal growth and function
7. Isolate clonal cells with growth advantages from aging cell populations using clonal methods and cultivate them for purification.
Considerations for U-2OS Transfection:
1. Ensure cells are in good condition and in the logarithmic growth phase, with optimal cell density typically between 70-80%.
2. Monitor digestion time to avoid excessive cell damage.
3. Ensure cells are dissociated into single cells to avoid clumping.
4. Ensure cell viability is ≥80% during experiments.
5. For electroporation, control the number of cells and seed into appropriate culture plates.
6. Post-electroporation, ensure an adhesion rate of ≥70%.
7. Control experimental timings during electroporation; the procedure should not be excessively prolonged.
8. Choose suitable lipid transfection reagents for U-2OS cells and determine optimal transfection conditions through preliminary experiments.
9. For lentivirus applications, maintain cell confluency at 30-40% prior to infection, to prevent excessive density.
10. Prior to formal experiments using viral methods, conduct preliminary tests to determine the most suitable MOI and add helper agents such as Polybrene pre-infection.
11. Ensure transfection reagents are fully mixed before use to ensure homogeneity.
12. It is recommended to conduct preliminary drug screening tests to find the optimal drug concentrations before proceeding with full-scale screenings post-transfection.
Considerations for U-2OS Clonal Expansion (Single Clone) Experiments:
1. Ensure cells are in good condition, with few senescent cells, and maintain approximately 70% confluency before expansion.
2. Ensure cell viability is ≥85% for seeding clones.
3. Conduct preliminary experiments to determine an appropriate clonal seeding density, avoiding too low a proportion of single clones.
4. Ensure even distribution of cells when seeding into 96-well plates.
5. When diluting cell counts, aim for concentrations between 1×10^6 and 2×10^6.
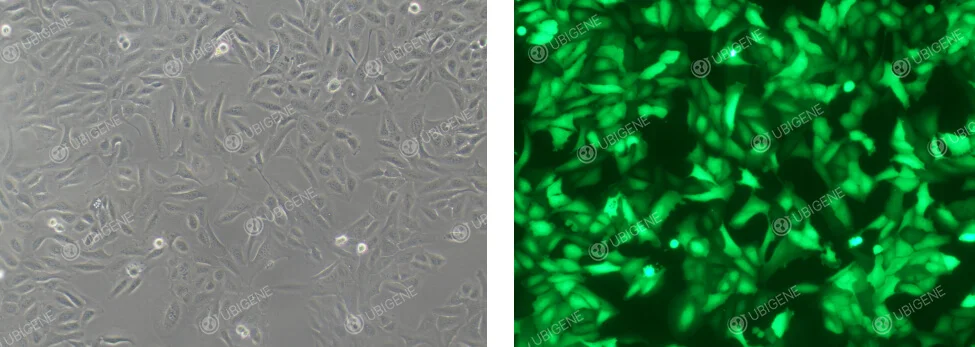
Images of lentivirus infection
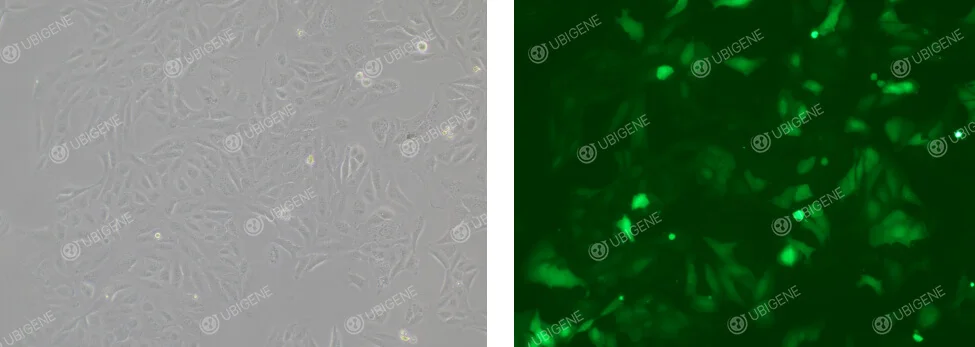
Images of electroporation method
Hot U-2OS KO Cells Available In-Stock
Starting at ¥4980
| Catalog # | Gene | Cell |
| YKO-H537 | PBK | U-2OS |
More U-2 OS cell-related products, please feel free to inquire!


