Expert Insights | Practical HEK293 Cell Culture and Gene-Editing Protocols


Expert Insights | Practical HEK293 Cell Culture and Gene-Editing Protocols
HEK293 cell line is a “regular guest” in the research community, frequently used in high-throughput library screening, viral packaging, and protein expression experiments. Their high transfection efficiency, stable expression capabilities, and well-established handling protocols make them a preferred choice across diverse experimental platforms. However, to ensure HEK293 cell line perform consistently and efficiently in your projects, attention to every operational detail is critical. In this article, Ubigene will guide you through practical strategies and key considerations—from cell thawing, passaging, and cryopreservation to transfection and single-cell cloning—helping you minimize experimental risks and streamline your research workflow.
Cell Line information
Cell Line: HEK293 (Human Embryonic Kidney Cell Line)
Morphology: Epithelial-like, adherent cells
Culture Medium: 90% DMEM + 10% FBS
Atmosphere: 95% air, 5% CO₂
Temperature: 37 °C
Medium Change Frequency: Every 2–3 days
Passage Ratio: 1:3–1:6
Characteristics of the normal cells: Normal HEK293 cells display irregular polygonal or spindle shapes with well-defined borders and a cobblestone-like arrangement in a confluent monolayer.

Characteristics of the Abnormal cells: Cells exhibit altered morphology, including blurred edges, elongation, and fibrotic appearance. Increased pseudopodia formation, vacuole or granule accumulation may be observed, and cell boundaries appear indistinct.

Cell Thawing
- 1. Preparation: Prepare 7 mL of complete culture medium in a centrifuge tube for later use.
- 2. Cell Thawing: Remove the vial from dry ice. Hold the cap with forceps and place the vial in a 37 °C water bath. Gently swirl until the ice is mostly melted (approximately 1 minute).Important: Do not submerge the cap in water.
- 3. Centrifugation: Transfer the thawed cell suspension to a centrifuge tube and centrifuge at 1100 rpm for 4 minutes. Carefully discard the supernatant.
- 4. Resuspension and Seeding: Resuspend the cell pellet in complete culture medium and seed into an appropriately sized culture dish or flask.
- 5. Cell Culture: Place the culture dish or flask in a 37 °C incubator. After 24 h, check for cell attachment and general cell appearance.
Cell Passaging (Example: T25 Flask)
- 1. Confluency Check and Washing: When cells reach 80–90% confluency, remove the culture medium in a biosafety cabinet. Wash the cells 1–2 times with 5 mL of PBS.
- 2. Trypsinization: Add 1 mL of trypsin-EDTA to the flask, gently swirl to ensure the solution covers all cells. Incubate in a 37 °C incubator for 1–2 minutes. Monitor under a microscope; when ≥80 % of the cells have rounded up, gently tap the flask. As soon as most cells detach, immediately neutralize the trypsin.
- 3. Neutralization: Add 2 mL of complete culture medium (twice the trypsin volume) to inactivate the trypsin, and transfer the cell suspension to a 15 mL centrifuge tube.
- 4. Centrifugation: Centrifuge at 1100 rpm at room temperature for 4 minutes. Discard the supernatant and resuspend the cell pellet in fresh complete medium.
- 5. Seeding: Split the cells at a 1:3–1:6 ratio into new flasks. Observe cell attachment and morphology the following day.
Cell Cryopreservation
- 1. Cell Collection: Harvest cells following the standard passaging procedure and transfer the detached cells into a centrifuge tube.
- 2. Centrifugation: Centrifuge at 1100 rpm for 4 minutes at room temperature and discard the supernatant.
- 3. Resuspension and Cryopreservation: Gently resuspend the cell pellet in freezing medium. Dispense 1 mL aliquots (1 × 10⁶ cells/mL) into labeled cryovials. Clearly mark each vial with the cell line name, passage number, and date.
- 4. Cooling and Storage: Place cryovials in a controlled-rate freezing container and store at –80 °C overnight. The following day, transfer the vials to liquid nitrogen for long-term storage.
Cell Culture Considerations
- · Culture Medium and Serum: Use the appropriate basal medium supplemented with the correct concentration of serum. Prepared complete medium should be stored at 4 °C and used within 2 weeks.
- · Culture Environment: Ensure that incubator conditions (37 °C, 5% CO₂, appropriate humidity) are properly maintained.
- · Pre-Warming Reagents: Pre-warm medium and trypsin to 37 °C to minimize thermal stress.
- · Passaging Timing: Passage cells when they reach 80–90% confluency, typically every 2–3 days.
- · Cell Passaging: Monitor trypsinization time and concentration carefully to avoid cell damage. Avoid excessive pipetting that may compromise cell membranes. If cell attachment is uneven, gently swirl the flask to distribute cells evenly.
- · Cells Not Adhering: Check serum quality, use coated culture vessels if necessary, or switch to 0.05% EDTA-containing trypsin (e.g., TrypLE™) with a shorter digestion time of 30–60 seconds.
Cell Transfection Considerations
- · Cell condition: Use cells in the logarithmic growth phase (70–80 % confluency) with ≥95 % viability (Trypan Blue exclusion).
- · Trypsinization Time: Avoid over-trypsinization to prevent cell damage during detachment.
- · Single-Cell Suspension: During the procedure, ensure cells are gently pipetted to form a single-cell suspension and prevent clumping.
- · Transfection Reagents: Mix transfection reagents thoroughly before use to ensure homogeneity.
- · Drug Selection Pre-Test: Perform a preliminary drug selection experiment to determine the optimal concentration for post-transfection selection.
- · Cell Electroporation Considerations
- (1) Cell Number: Carefully control the number of cells used for electroporation. After electroporation, seed cells into appropriately sized culture vessels based on the cell quantity.
- (2) PBS Washing: Wash cells 1–2 times with PBS to completely remove residual serum, preventing ionic interference during electroporation.
- (3) Parameter Optimization: Perform preliminary experiments to optimize electroporation parameters for your specific setup.
- (4) Post-Electroporation Attachment: Ensure that at least 70% of cells successfully attach to the culture surface after electroporation.
- (5) Time Management: Control the duration of the electroporation procedure; avoid unnecessarily long exposure times to reduce cellular stress.
- · Lentiviral Transduction Considerations
- (1) MOI Optimization: Perform preliminary experiments to determine the optimal multiplicity of infection (MOI) before the formal experiment.
- (2) Cell Confluency: Maintain cells at 30–40% confluency prior to viral infection; avoid higher confluency.
- (3) Polybrene Addition: Add Polybrene before infection to enhance viral entry.
- (4) Culture Medium Change: Replace the culture medium 24 hours post-infection to remove residual virus and reduce cytotoxicity.
- (5) Virus Handling: Avoid repeated freeze–thaw cycles of the viral stock to preserve viral activity.
- · Lipid-Based Transfection Considerations
- (1) Reagent Selection and Optimization: Choose a lipid-based transfection reagent suitable for HEK293 cells. Perform preliminary experiments to determine optimal transfection conditions.
- (2) Cell Seeding and Confluency: Plate cells 24 hours before transfection. Maintain 60–70% confluency at the time of transfection.
- (3) DNA–Reagent Complex Formation: First dilute DNA in Opti-MEM, then add the lipid reagent; adding DNA to the lipid will compromise transfection efficiency.
- (4) Complex Incubation: Incubate DNA–lipid complexes at room temperature for 15–20 min (<10 min gives incomplete complexes; >30 min increases cytotoxicity).
- (5) Addition to Cells: Add complexes dropwise along the edge of the culture vessel and gently swirl to mix, avoiding direct impact on the cells.
Single-Cell Cloning Considerations
- · Cell Growth Stage: Use cells in the logarithmic growth phase for seeding. Aim for ~70% confluency before starting the single-cell cloning.
- · Cell Viability: Ensure cell viability is ≥90% prior to seeding.
- · Reagent Preparation: Pre-warm all reagents, including culture medium, trypsin (or alternative), and PBS.
- · Gentle Dissociation: Use mild dissociation reagents (e.g., TrypLE Express) to minimize cell stress.
- · Seeding Density Optimization: Perform preliminary experiments to determine an appropriate seeding gradient, avoiding excessively low single-cell clone numbers.
- · Uniform Distribution: When seeding into 96-well plates, ensure cells are evenly distributed. Fill the outer wells with PBS to reduce edge effects and evaporation.
- · Dilution Strategy: Use serial dilutions; after counting, target ~1–2 × 10xcells per well to obtain well-separated single-cell clones.
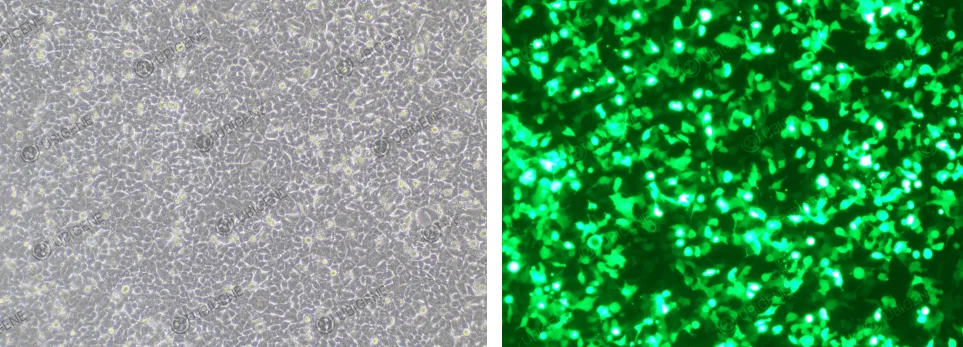
Cell images (Electroporation transfection method)
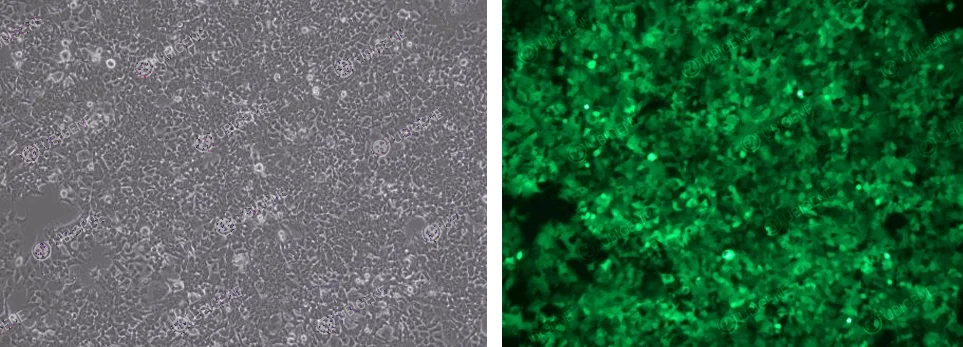
Cell images (Lentivirus Infection method)



