Annual Review | 2025 Collection of High-Impact Publications Citing Ubigene


In life science research, gene editing technologies continue to drive breakthroughs across multiple disciplines, with robust and reliable technical support serving as the foundation of high-quality scientific output. Ubigene has been deeply engaged in the field of gene editing, providing end-to-end products and services to over ten thousand research institutions and enterprises worldwide. The reliability of its technologies has been extensively validated by a large body of high-impact scientific publications.
In 2025, Ubigene continued to empower scientific innovation, witnessing and contributing to the achievement of multiple milestone discoveries that fully demonstrate the scientific value of its products and services. Over the course of the year, Ubigene supported the publication of 186 high-quality research articles, with a cumulative impact factor exceeding 1,000. These studies involved a broad portfolio of offerings, including knockout (KO) cell lines, 、 point mutation cell lines, 、 the EZ-Stem™ stem cell series products, 、 luciferase (Luc) reporter cell lines, and related gene editing services.Notably, several of these studies were published in leading international journals such as Signal Transduction and Targeted Therapy, Cell, and Advanced Science, underscoring Ubigene’s strong technical expertise and sustained value in high-end research applications. To date, Ubigene has supported the publication of more than 400 high-quality scientific papers, continuously advancing life science research through reliable gene editing technologies and ongoing product innovation.
To highlight key research trends in gene editing in 2025 and provide valuable references for the scientific community, we have compiled a collection of high-impact publications that cite Ubigene’s products and services. These studies span multiple research areas, including virology, oncology, and beyond. In the following sections, we explore the scientific innovation and translational significance of these representative research achievements.
| Category | Journal | Impact Factor |
| Gene Knockout Cell Line Services | Signal Transduction and Targeted Therapy | 52.7 |
| Advanced Materials | 26.8 | |
| Science Bulletin | 21.1 | |
| Signal Transduction and Targeted Therapy | 52.7 | |
| ACS Nano | 16 | |
| Redox Biology | 11.9 | |
| Gene Point Mutation Cell Line Services | Advanced Science | 14.1 |
| Wild-Type Cell Lines | Cellular & Molecular Immunology | 19.8 |
| Luciferase (Luc) Reporter Cell Lines | Nature Communications | 15.7 |
| EZ-Stem™ Stem Cell Series Product | Cell | 42.5 |
| Cas9 Protein | Advanced Functional Materials | 19 |
| EZ-Editor™ Gene Editing Series Product | Nature Communications | 15.7 |
Table 1. Overview of Selected High-Impact Publications
High-Impact Publications in Gene Knockout Cell Line Services
Gene Knockout Cell Lines
1.Title: Ninjurin-1 mediates cell lysis and detrimental inflammation of PANoptosis during influenza A virus infection
Journal: Signal Transduction and Targeted Therapy
Impact Factor: 52.7
Cited Products / Services: NINJ1 Knockout cell line (A549) 、 NINJ1 Knockout cell line (THP-1)
Article Abstract: Influenza A virus (IAV) induces ZBP1-mediated PANoptosis, a lytic and inflammatory form of programmed cell death characterized by the coordinated activation of apoptotic, necroptotic, and pyroptotic pathways. Ninjurin-1 (NINJ1) has recently been identified as a key mediator of plasma membrane rupture and has been shown to exert diverse functions across different types of cell death. However, the role of NINJ1 in IAV-induced PANoptosis and viral pneumonia remains unclear.In this study, we demonstrate that IAV infection induces the upregulation of NINJ1 expression, followed by its oligomerization and subsequent mediation of cell lysis in infected macrophages. Loss of NINJ1 effectively blocks plasma membrane rupture as well as the release of damage-associated molecular patterns (DAMPs) and interleukin-1β (IL-1β), without affecting the execution of cell death itself. Activation of any single PANoptotic pathway is sufficient to trigger NINJ1 oligomerization and robust cell lysis. Correspondingly, only simultaneous inhibition of all PANoptotic pathways can prevent NINJ1 oligomerization, cell death, and membrane rupture.
In vivo, NINJ1 deficiency markedly alleviates IAV-induced lung injury and reduces mortality. Furthermore, we identify an association between elevated NINJ1 expression and poor clinical outcomes in patients with COVID-19. Collectively, these findings establish a critical role for NINJ1 in the immunopathology of IAV infection and suggest that NINJ1 may serve as a potential biomarker for disease severity and prognosis in viral pneumonia and viral sepsis.
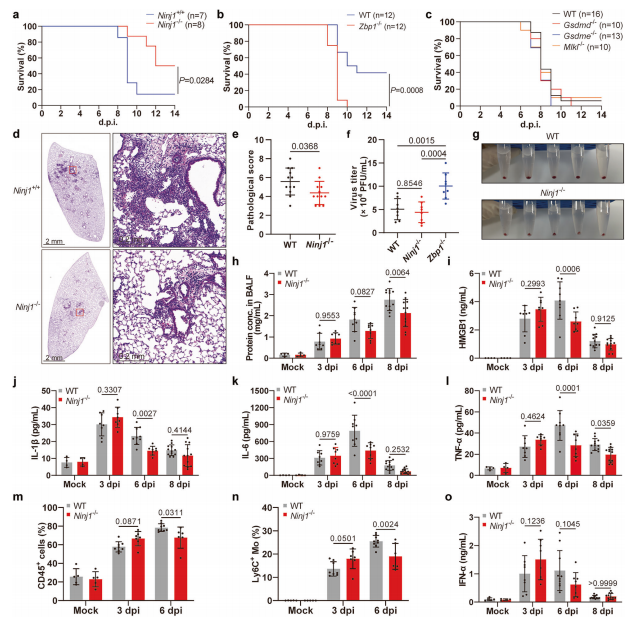
Figure 1. Selected Results from the Study
2.Title: Peptide Amphiphiles Hitchhike on Endogenous Biomolecules for Enhanced Cancer Imaging and Therapy
Journal: Advanced Materials
Impact Factor: 26.8
Cited Products / Services: LDL-R Knockout cell line (Hela)
Article Abstract: Interactions between nanomaterials and endogenous biomolecules in vivo critically determine their biological fate. This study demonstrates that self-assembled peptide amphiphile (PA) nanostructures dynamically interact with endogenous biomolecules and exploit native physiological processes to target a broad range of solid tumors. In the circulation, self-assembled PA nanostructures predominantly associate with lipoproteins through disassembly and reassembly, thereby prolonging blood circulation time and significantly enhancing drug accumulation and retention at tumor sites.Mechanistic investigations reveal that PAs are internalized through assembly with the cancer cell membrane via a receptor-independent process. Leveraging this interaction, the PA developed in this study—self-assembled glutamic acid (SA-E)—exhibits selective tumor enrichment across multiple xenograft, syngeneic, patient-derived xenograft (PDX), and transgenic rodent models. Moreover, SA-E enables the precise delivery of potent chemotherapeutic agents to both syngeneic and xenograft tumors, while markedly reducing systemic toxicity.
Owing to its simple modular design and broadly applicable tumor accumulation mechanism, SA-E represents a promising platform with significant potential for cancer imaging and therapeutic applications.
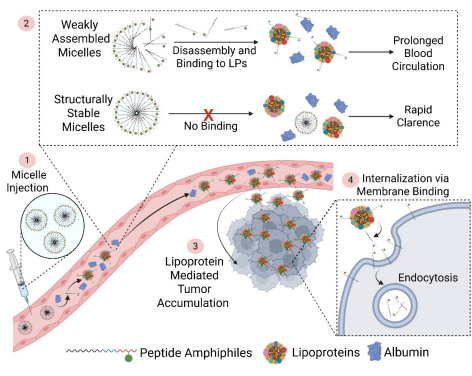
Figure 2. Schematic Illustration of the Proposed Mechanism
3.Title: Structural insight into GPR155-mediated cholesterol sensing and signal transduction
Journal: Science Bulletin
Impact Factor: 21.1

Cited Products / Services: GPR155 Knockout cell line (HEK293)
Article Abstract: Cholesterol (CHL) is a fundamental building block of membrane biogenesis and serves as a precursor for oxysterols, steroid hormones, bile acids, and vitamin D. Lysosomes function as the primary sorting station for low-density lipoprotein (LDL), which transports dietary cholesterol, and also serve as the activation site of the mechanistic target of rapamycin complex 1 (mTORC1), a central kinase regulating cellular growth. Recent studies have reported that the lysosomal transmembrane protein GPR155 signals cholesterol sufficiency to mTORC1 by sequestering the GTPase-activating protein complex GATOR1 at Rag GTPases.Although recently resolved structures of GPR155 have revealed its cholesterol-binding site, how signals are transmitted from this site to the soluble region of GPR155 and subsequently to GATOR1 remains unclear. By determining cryo–electron microscopy (cryo-EM) structures of GPR155–CHL complexes in three distinct conformational states, complemented by long-timescale molecular dynamics simulations, we observed dynamic rearrangements across multiple domains. Cholesterol binding induces an expansion of the cleft between the transporter domain and the GPCR domain.
Notably, a previously unresolved N-terminal extension of transmembrane helix 16 (TM16) functions as a mechanical lever, transmitting cholesterol-induced rotational motion of the GPCR domain to the soluble region of GPR155. This work not only elucidates how GPR155 senses cholesterol, but also reveals a deeper mechanistic principle—how signals detected within the transmembrane region are relayed to the luminal/extracellular domain (LED) and the DEP domain.
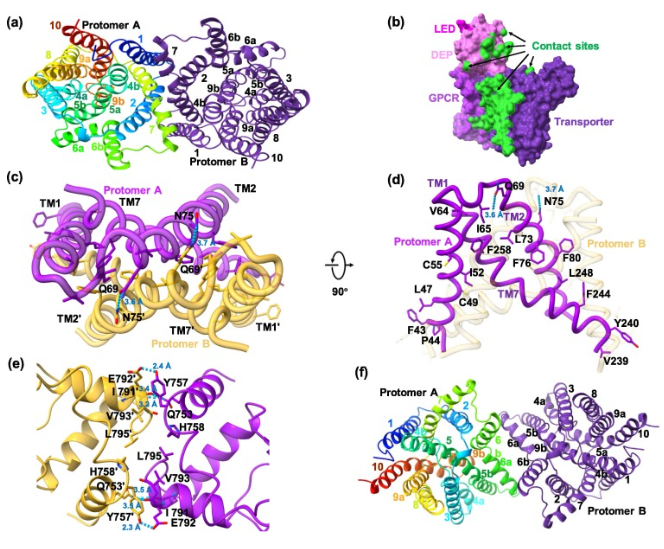
Figure 3. Selected Results from the Study
Gene Knockout Lentivirus
1.Title: A donor PD-1+CD8+ TSCM-like regulatory subset mobilized by G-CSF alleviates recipient acute graft-versus-host-disease
Journal: Signal Transduction and Targeted Therapy volume
Impact Factor: 52.7
Cited Products / Services: hBCL6 CRISPR/Cas9 knockout lentiviral vector
Article Abstract: Donor selection is a critical determinant of acute graft-versus-host disease (aGVHD) following allogeneic hematopoietic stem cell transplantation (allo-HSCT). To optimize current clinical donor selection criteria and identify donor lymphocyte subsets associated with improved recipient outcomes, we analyzed CD4⁺ and CD8⁺ T-cell subsets in granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood from 80 donors and evaluated the incidence of aGVHD in 80 corresponding recipients undergoing haploidentical or matched allo-HSCT. G-CSF–induced expansion of T-cell subsets varied substantially among donors.We identified a previously unrecognized PD-1⁺CD8⁺CD45RA⁺CCR7⁺ T-cell subset in qualified donors that was significantly associated with a reduced incidence of aGVHD and enhanced post-transplant anti-infective immunity. The protective effect of this subset against aGVHD was validated in an independent cohort (n = 30). Single-cell RNA sequencing revealed that this subset exhibits a stem cell–like memory T-cell (TSCM) transcriptional profile and possesses dual regulatory T-cell (Treg) and effector T-cell (Teff) functional characteristics, suggesting a bifunctional role in suppressing aGVHD while preserving graft-versus-leukemia (GVL) activity.
Interestingly, following G-CSF mobilization, donor PD-1⁺CD8⁺ TSCM-like regulatory cells upregulated PD-1 expression in a BCL6-dependent manner. Furthermore, the murine counterpart of this subset (PD-1⁺CD8⁺CD44⁻CD62L⁺) was shown to alleviate aGVHD, and the presence of this population was confirmed in clinical transplant recipients. Collectively, this study identifies a novel donor-derived peripheral T-cell subset that simultaneously suppresses aGVHD and promotes immune reconstitution after transplantation. This subset may serve as a valuable biomarker for selecting optimal haploidentical and matched donors. Notably, owing to their combined Treg- and Teff-like functions, these cells also represent a promising therapeutic avenue not only for aGVHD but potentially for autoimmune diseases.
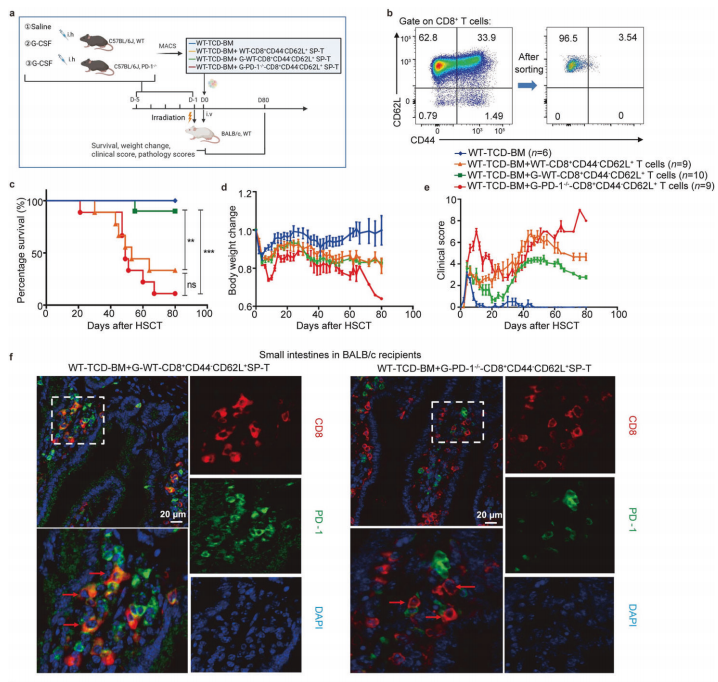
Figure 4. Selected Results from the Study
2.Title: Emerging of Ultrafine Membraneless Organelles as the Missing Piece of Nanostress: Mechanism of Biogenesis and Implications at Multilevels
Journal: ACS Nano
Impact Factor: 16

Cited Product/Service: The lentivirus (CMV promoter) carrying CRISPR/Cas9 System, G3BP1-sgRNA, G3BP2-sgRNA, and G3BP1-fused with BFP/EGFP was all purchased from Ubigene technology and used as received
Article Abstract: Understanding the interactions between nanomaterials and cellular structures is crucial for the biomedical application of nanoparticles. In this study, we identified a subtype of stress granules induced by gold nanorods (AuNRs), termed nanomaterial-induced stress granules (NSGs). These NSGs exhibit distinct physical and functional characteristics compared to conventional stress granules. Uptake of AuNRs triggers intracellular reactive oxygen species (ROS) accumulation and protein misfolding, leading to NSG formation. Structurally, NSGs feature a gel-like core and a liquid outer shell, positively regulated by heat shock protein 70 (HSP70) and negatively regulated by heat shock protein 90 (HSP90) and the ubiquitin-proteasome system. AuNRs promote NSG assembly through interaction with G3BP1, reducing the energy required for liquid-liquid phase separation. NSGs modulate cellular functions by influencing mRNA surveillance mechanisms and activating the AMP-activated protein kinase (AMPK) signaling pathway, which is essential for cellular stress responses. This work highlights the critical role of liquid-liquid phase separation (LLPS) in nanomaterial metabolism and suggests nanodroplets as potential targets for drug delivery strategies, advancing the field of nanomedicine.
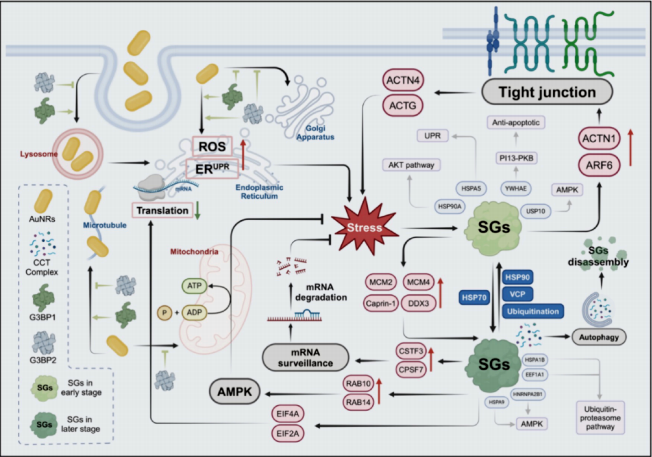
Figure 5. Mechanistic Illustration of Nanomaterial-Induced Stress Granule (NSG) Formation.
Gene Knockout Plasmid
Title: Knockout of the sulfide: quinone oxidoreductase SQR reduces growth of HCT116 tumor xenograft
Journal: Redox Biology
Impact Factor: 11.9

Cited Product/Service: YKO-RP006-hRABL6 [gRNA] plasmids
Article Abstract: Colorectal cancer (CRC) exhibits significant heterogeneity and diversity, necessitating the development of novel therapeutic targets. Polysulfides have been associated with CRC progression and immune evasion, though the underlying mechanisms remain incompletely understood. Sulfide:quinone oxidoreductase (SQR), a mitochondrial flavoprotein, catalyzes the oxidation of hydrogen sulfide (H₂S) and the generation of polysulfides. This study investigated the role of SQR in CRC pathogenesis and its potential as a therapeutic target. SQR knockout disrupted polysulfide homeostasis in HCT116 CRC cells, impaired mitochondrial function, inhibited cell proliferation, and induced early apoptosis. In vivo, SQR knockout significantly reduced tumor volume in a colon xenograft mouse model. Although transcription of glycolytic genes remained largely unchanged, metabolomic analysis revealed reprogramming of the fructose-1,6-bisphosphate degradation step, catalyzed by pyruvate kinase A. Western blot and enzymatic activity assays confirmed decreased ALDOA expression and activity. Collectively, these findings establish SQR as a key regulator of mitochondrial function and metabolic control in CRC, with its knockout inducing metabolic reprogramming and suppressing tumor growth in HCT116 xenografts, providing a foundation for SQR-targeted therapies in colorectal cancer.
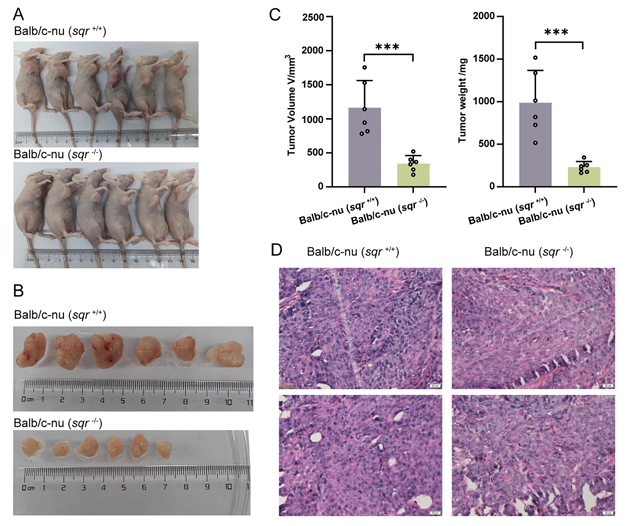
Figure 6. Selected Results from the Study
High-Impact Publications Using Point Mutation Cell Lines
Title: Colorectal Cancer Cells–Derived Exosomal PIK3CA Mutation DNA Promotes Tumor Metastasis by Activating Fibroblast and Affecting Tumor Metastatic Microenvironment
Journal: Advanced Science
Impact Factor: 14.1

Cited Product/Service: Gene editing of LS174T cells carrying the PIK3CA H1047R mutation
Article Abstract: Exosomes contribute to the formation of the tumor metastatic microenvironment (TME) by transferring tumor-specific molecules. However, most studies have focused on exosomal RNA and proteins, with exosomal DNA remaining largely unexplored. This study demonstrates that exosomal DNA containing the PIK3CA H1047R mutation, derived from colorectal cancer (CRC) cells, can be delivered to recipient fibroblasts. Upon transcription and translation, this mutant DNA interacts with the endogenous P85 regulatory subunit of the phosphoinositide 3-kinase (PI3K) pathway, driving fibroblast conversion into cancer-associated fibroblasts (CAFs). These CAFs secrete high levels of IL-6, promoting tumor cell migration in vitro and accelerating lung metastasis in vivo. PIK3CA H1047R mutations were detected in CAFs within both primary tumors and metastatic lesions, suggesting that this mutation may facilitate metastasis by reshaping the tumor microenvironment. Clinically, CRC patients harboring PIK3CA H1047R mutations with elevated IL-6 levels exhibit a significantly increased risk of metastasis. These findings indicate that combined detection of exosomal PIK3CA H1047R and serum IL-6 levels may serve as a promising diagnostic and prognostic tool, while simultaneous targeting of PIK3CA H1047R and IL-6 represents a potential therapeutic strategy for colorectal cancer.
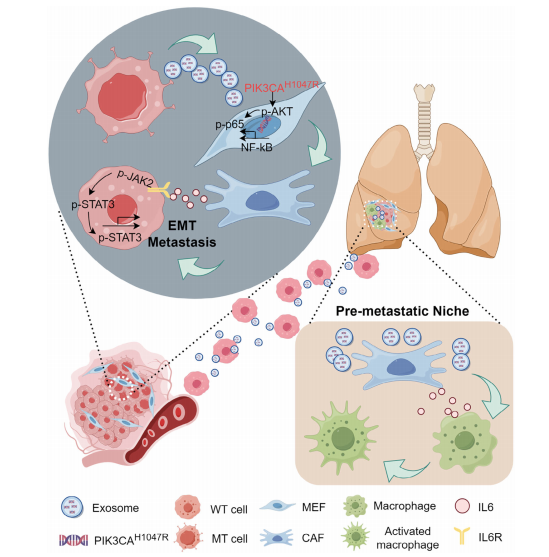
Figure7. Schematic Illustration of the Proposed Mechanism
High-Impact Publications Using Wild-Type Cells
Title: GPNMB disrupts SNARE complex assembly to maintain bacterial proliferation within macrophages
Journal: Cellular & Molecular Immunology
Impact Factor: 19.8

Cited Product/Service: THP-1 cells
Article Abstract: Phagocytosis plays a critical role in controlling bacterial growth within macrophages. However, the mechanisms regulating autophagosome-lysosome fusion during bacterial infection remain incompletely understood. Using leprosy as a model, this study investigates the interplay between host defense mechanisms and bacterial infection. Glycoprotein non-metastatic melanoma protein B (GPNMB) is highly expressed in macrophages from lepromatous leprosy (L-Lep) patients and interferes with xenophagy during infection. Mechanistically, GPNMB interacts with STX17 on autophagosomes, leading to reduced N-glycosylation at the N296 site of GPNMB. This modification promotes degradation of SNAP29, thereby blocking assembly of the STX17-SNAP29-VAMP8 SNARE complex. Consequently, autophagosome-lysosome fusion is impaired, inhibiting autophagic flux. Beyond Mycobacterium leprae, GPNMB deficiency restricts the proliferation of multiple intracellular bacteria in human macrophages, suggesting a broad role in intracellular bacterial infections. In vivo, Gpnmb^fl/fl Lyz2-Cre mice show significantly reduced Mycobacterium leprae load compared to controls. Overall, this study uncovers a previously unrecognized function of GPNMB in host antibacterial defense and elucidates its regulatory role in SNARE complex assembly.
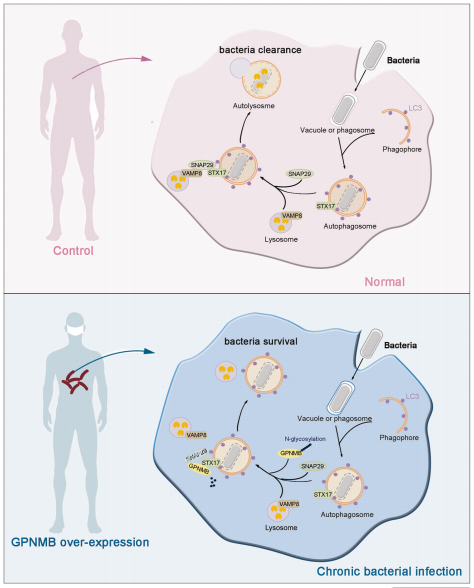
Figure 8. Schematic Illustration of the Proposed Mechanism
High-Impact Publications Using Luc Stale Cells
Title: Engineering nanozyme immunomodulator with magnetic targeting effect for cascade-enzyodynamic and ultrasound-reinforced metallo-immunotherapy in prostate carcinoma
Journal: Nature Communications
Impact Factor: 15.7

Cited Product/Service: Luc-RM-1 cell line
Article Abstract: Traditional immunotherapies often show limited efficacy due to the immunosuppressive characteristics of the tumor microenvironment (TME). To overcome this limitation, this study developed ZFPG nanoparticles (ZFPG NPs) composed of a ZnFe₂O₄@Pt core and a glucose oxidase (GOx) shell. These nanoparticles exhibit five enzymatic activities, excellent sonosensitivity, and superior magnetic targeting capabilities, enabling combined sonodynamic-metallo-immunotherapy in male mouse prostate cancer models. Magnetic targeting significantly enhances nanoparticle accumulation at tumor sites while preserving minimal off-target deposition in liver and kidney tissues. The multi-enzyme cascade catalysis and sonosensitive properties efficiently deplete glutathione and glucose, augmenting hydrogen peroxide production and utilization, thereby triggering multiple bursts of reactive oxygen species (ROS). These effects upregulate heme oxygenase 1 (HMOX1), promoting Fe²⁺ and lipid peroxide accumulation, which induces immunogenic ferroptosis. This strategy remodels the immunosuppressive TME and inhibits lung metastasis, effectively enhancing antitumor immunity. Overall, the synergistic approach provides a potent solution to overcome the limitations of conventional immunotherapies.
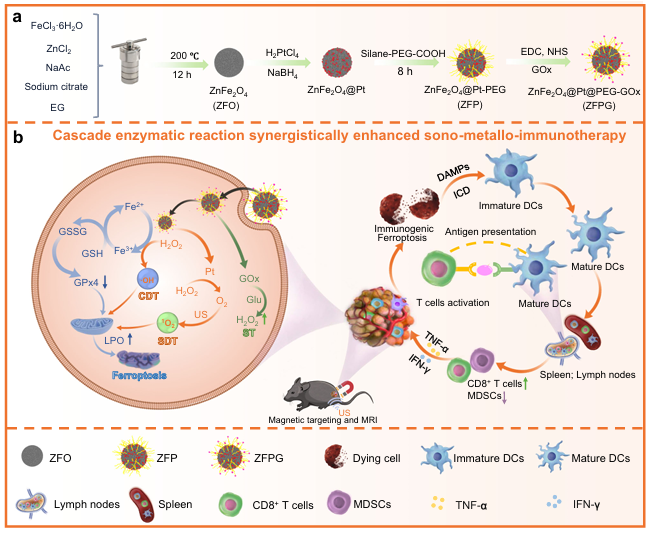
Figure 9. Schematic Illustration of the Proposed Mechanism
High-Impact Publications Using EZ-Stem™ Stem Cell Products
Title: Hyperacute rejection-engineered oncolytic virus for interventional clinical trial in refractory cancer patients
Journal: Cell
Impact Factor: 42.5

Cited Product/Service: EZ-Stem™ Cell Culture Medium
Article Abstract: Oncolytic virus (OV) therapy has shown tremendous potential for treating malignant tumors in recent years. However, intravenous administration faces two major limitations: safety concerns and inherent immune deficiencies. In this study, a recombinant Newcastle disease virus carrying the pig α1,3-galactosyltransferase gene (NDV-GT) was developed to induce hyperacute rejection. Preclinical studies validated the feasibility of this approach. Using a CRISPR-mediated primary hepatocellular carcinoma monkey model, intravenous NDV-GT demonstrated robust tumor cell clearance. Importantly, an interventional clinical trial involving 20 patients with relapsed or refractory metastatic cancers (WHO China Clinical Trial Registration: ChiCTR2000031980) showed a disease control rate of 90%, durable efficacy, and no severe adverse events or clinically significant neutralizing antibodies. These results confirm the extremely low immunogenicity of NDV-GT under the tested conditions and support its feasibility for immunovirotherapy. Overall, this study demonstrates the high safety and efficacy of intravenous NDV-GT, providing an innovative platform for tumor therapy and other viral vector-based applications.
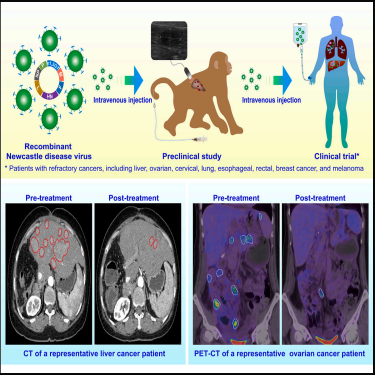
Figure 10. Graphical Abstract
High-Impact Publications Using Cas9 Protein
Title: Intelligent Nano-Cage for Precision Delivery of CRISPR-Cas9 and ACC Inhibitors to Enhance Antitumor Cascade Therapy Through Lipid Metabolism Disruption
Journal: Advanced Functional Materials
Impact Factor: 19

Cited Product/Service: Cas9-EGFP fusion protein
Article Abstract: Reprogramming of lipid metabolism is a hallmark of cancer, and targeting key enzymes in fatty acid synthesis, such as acetyl-CoA carboxylase 1 (ACC1), represents a promising therapeutic strategy. In this study, peptide-modified red blood cell membrane-coated nanocages (NTA630-NCs-RBCM-T) were engineered to co-deliver CRISPR-Cas9 and ACC1 inhibitors, achieving dual suppression of ACC1 and optimizing antitumor cascade therapy efficacy. The nanocages efficiently targeted cancer cells via peptide-mediated delivery and released the Cas9 complex and ND630 in response to lysosomal enzymes, proton sponge effect, and elevated glutathione levels. Cas9-mediated knockout of ubiquitin-specific protease 22 (USP22), combined with direct ACC1 inhibition by ND630, simultaneously blocks fatty acid synthesis pathways, enhances polyunsaturated fatty acid (PUFA) uptake, and induces lipid peroxidation. This leads to tumor cell death, particularly via ferroptosis and apoptosis, demonstrating potent antitumor activity.
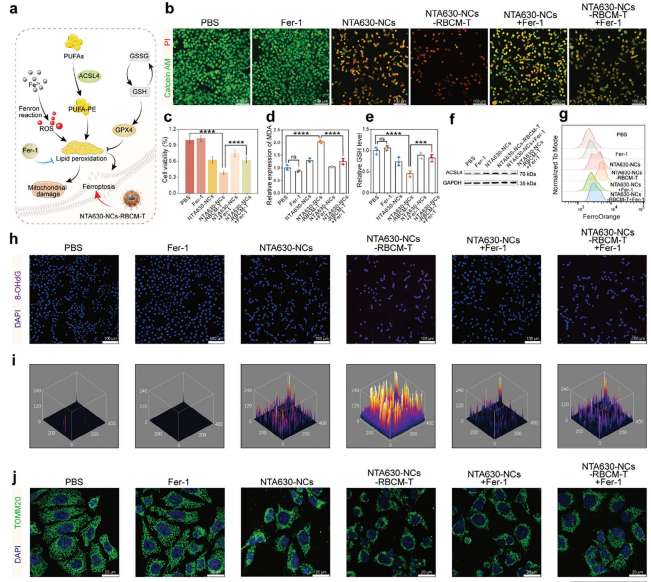
Figure 11. Article Partial Results Figure
High-Impact Publications Using EZ-editor™ Gene Editing Series Products
Title: Auricular malformations are driven by copy numbervariations in a hierarchical enhancer cluster and a dominant enhancer recapitulates human pathogenesis
Journal: Nature Communications
Impact Factor: 15.7

Cited Product/Service: EZ-editor™ Monoclone Genotype Validation Kit
Article Abstract: Enhancers, through coordinated action of transcription factors (TFs), determine the spatial specificity and expression levels of target genes, and their dysregulation can lead to disease. Although downstream enhancers of HMX1 have been associated with auricular malformations, the mechanisms underlying bilateral concha ear (BCE) anomalies remain unclear. This study identified a copy number variation (CNV) encompassing three enhancers—termed the Positional Identity Hierarchical Enhancer Cluster (PI-HEC)—which drives BCE by coordinating regulation of HMX1 expression. Each enhancer exhibits distinct activity-localization-structure features, with the dominant enhancer modulating activity and specificity via high-mobility group (HMG) box-mediated interaction with coordinating factors and homeodomain transcription factor motifs. Mouse models confirmed that abnormal Hmx1 expression in neural crest-derived fibroblasts at the auricular base, coupled with ectopic expression in distal auricular regions, disrupts outer ear development, affecting cartilage, muscle, and epidermal formation. This study elucidates the mechanisms of mammalian auricular morphogenesis and highlights the complex intra-enhancer and enhancer-TF cooperative regulation.
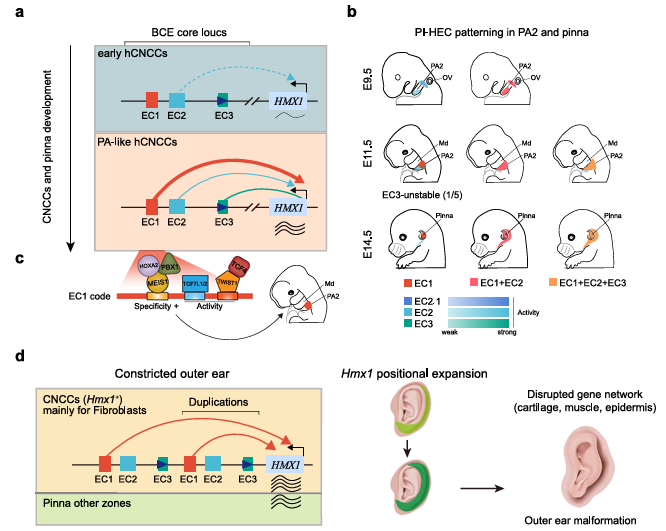
Figure 12. Mechanistic Diagram
Ubigene in 2026: Advancing Technology and Service Excellence
In the new year, Ubigene will continue to deepen its technological expertise and refine its services, looking forward to continuing this journey with you. If you have any needs for gene editing products or services, please feel free to reach out to us at any time!
For researchers seeking to further enhance experimental efficiency and data reliability, or requiring tailored technical support for specific research needs, Ubigene offers professional guidance and services to ensure optimal results.

