Achieving First-Time Success: Optimization Strategies for Gene Knockout Experiments in Cells


Achieving First-Time Success: Optimization Strategies for Gene Knockout Experiments in Cells

Gene knockout (KO), as a pivotal tool for elucidating gene function, investigating disease mechanisms, and validating drug targets, has been widely applied in life science research. However, in practice, this technology still faces critical challenges, including low knockout efficiency, significant off-target effects, and difficulties in establishing reliable validation systems, which limit the depth and robustness of the studies.
With extensive experience in gene editing, Ubigene offers a comprehensive and in-depth analysis of strategies to optimize gene knockout experiments across five key areas: essential technical points in experimental design and execution, core considerations, common technical challenges and misconceptions, typical issues and their solutions, and practical troubleshooting tips. This serves as a professional technical reference for researchers.
Tips & Strategies: Maximizing KO Success from the Outset
Precise sgRNA Design
- · Prioritize target sites within the 5' region of exons to maximize the likelihood of frameshift mutations, ensuring premature termination of protein translation.
- · Employ multiple computational tools (e.g., CRISPR Design Tool, Benchling, CHOPCHOP) for sgRNA scoring and off-target prediction. Integrate the results to select candidates with specificity scores ≥90, effectively minimizing off-target effects.
- · Consult species-specific functional genomic databases (e.g., Ensembl, UCSC Genome Browser) to avoid highly conserved domains or regions of unknown function, reducing potential compensatory effects.
Ubigene's proprietary Red Cotton CRISPR Gene Editing Designer can generate three ready-to-use KO cell line solutions within one minute. These tools allow rapid, reliable evaluation of gene knockout risk, making experimental design smarter, faster, and more robust.
Choosing the Appropriate Editing System and Vector
- · For conventional immortalized cell lines (e.g., HEK293, HCT116)plasmid-based co-transfection of Cas9/sgRNA is typically sufficient, offering a balance of cost-effectiveness and high efficiency.
- · For hard-to-transfect cells (e.g., neurons), lentiviral vectors for stable expression or RNP (ribonucleoprotein) delivery systems are recommended. Lentiviral vectors enable long-term editing through integration, while RNP delivery minimizes DNA integration-related cytotoxicity, enhancing cell viability.
- · For conditional knockout experiments, loxP/Cre recombination systems or inducible expression systems such as Tet-on/off should be employed to achieve spatiotemporal control of gene deletion, avoiding embryonic lethality or early developmental defects.
Ubigene has successfully achieved high-efficiency gene editing in over 300 cell types, completing more than 6,000 successful cases. Leveraging extensive experience and optimized workflows, we can significantly increase the recovery rate of positive clones while minimizing off-target effects and experimental failure. Knockout efficiency can reach up to 97%. Whether working with conventional cell lines, stem cells, or challenging cell types, Ubigene provides efficient, reliable, and reproducible customized gene editing solutions, empowering research teams to accelerate functional genomics studies, disease mechanism exploration, and drug target validation.
Establishing a Multi-Dimensional Validation System
- · DNA Level – PCR Amplification + Sequencing: Amplify the target region via PCR followed by Sanger sequencing or NGS analysis to assess indel (insertion/deletion) efficiency and mutation types.
- · Transcript Level: Use RT-qPCR to measure the relative expression of the target gene's mRNA, evaluating transcript degradation or truncation.
- · Protein Level: Perform quantitative analysis of protein expression via Western blot, or examine protein localization changes through immunofluorescence, providing direct confirmation of the knockout effect.
Ubigene's knockout cell line services include PCR detection and Sanger sequencing validation to validate the KO cells based on DNA level. For specialized validation needs, such as protein-level verification via Western blot, customized evaluation and services can be provided based on project requirements. Click here for online consultation >>>
Experimental Considerations: Attention to Detail Determines Success
Rigorous Quality Control of Cell Status
It is essential to maintain cells in optimal condition throughout the experiment. Avoid using high-passage cells or cells exhibiting abnormal morphology or reduced proliferation rates. High-passage cells may accumulate genomic variations, while suboptimal cell health can significantly decrease transfection efficiency and the activity of the editing system, ultimately compromising experimental reproducibility.
Standardized Handling of Reagents and Culture Media
Before transfection, ensure that culture media, transfection reagents, and related buffers are sterile and freshly prepared. Degraded reagents (e.g., inactivated growth factors in serum) or contamination (bacterial or mycoplasma) can induce cytotoxicity and interfere with the gene editing process.
Optimizing Transfection Conditions for Each Cell Type
Optimize transfection parameters according to the specific cell type, including DNA/reagent ratios, cell density, and pulse settings, to maximize editing efficiency and cell viability.
Proactive Design of Validation Systems and Controls
Before performing genome editing in cells, plan ahead for validation methods as well as positive and negative controls.
Common Challenges in Experiments and Their Solutions
| Challenges | Potential Reasons | Solutions |
|---|---|---|
| Low knockout efficiency | Insufficient sgRNA activity or low transfection efficiency | Redesign the target site, enhance delivery efficiency, or switch the editing system |
| High off-target rate | Poor sgRNA specificity | Optimize the design and use high-fidelity Cas9 (HiFi Cas9) |
| Gene knockout induces cell death | Target gene is essential | Use inducible knockout (cKO) or partial gene silencing via RNAi |
| Failure in Single-cell cloning | Low sorting efficiency or poor cell condition | Use an automated single-cell sorter and optimize culture conditions |
Typical Pitfalls and Prevention Strategies
Mistake 1: Overlooking Cell Type Differences
Using a uniform editing system and delivery method across all cell lines.
- Potential Risk: Hard-to-transfect cells (e.g., neuronal cells) may exhibit editing efficiencies below 10%, while sensitive cell lines may experience significant cell death due to vector toxicity, compromising experimental reproducibility.
- Prevention Strategy: Select an appropriate delivery system tailored to the specific cell type, and perform pilot experiments to evaluate the compatibility and efficiency of different systems.
Mistake 2: Overreliance on Predictive Software
- Potential Risk: In silico predictions may not fully capture the complexity of the cellular environment, resulting in knockout efficiencies below 30% or increased off-target effects.
- Prevention Strategy: Conduct small-scale pilot tests for candidate sgRNAs, measuring editing efficiency using methods such as restriction enzyme assays or flow cytometry. Complement this with off-target site sequencing to identify the most effective sgRNA, minimizing resource waste in large-scale experiments.
Mistake 3: Improper Timing of Validation
- Potential Risk: Detecting edits too early (within 24 hours post-transfection) may yield false negatives because editing is incomplete, whereas delayed detection (beyond 7 days) can dilute edited cells through proliferation of unedited cells, also producing false negatives.
- Prevention Strategy: Set an appropriate detection window based on the editing system. For transient transfections, assess 48–72 hours post-transfection, when Cas9 activity peaks and cells remain healthy. For stable knockout lines, evaluate 1–2 weeks after selection to ensure that surviving clones are genuinely edited.
Frequently Asked Questions
Q1. How long does it take to complete a KO cell line project?
The timeline depends on the cell type and knockout strategy. For standard cell lines, projects typically take 8–10 weeks, while more challenging cell types may require longer. Leveraging Ubigene's optimized workflow and the Red Cotton CRISPR Gene Editing Designer, positive clones can be identified in as little as 4 weeks, enabling researchers to accelerate their experimental progress.
Q2. Can difficult-to-transfect cells be edited?
Yes. CRISPR-U™ is Ubigene's proprietary high-efficiency gene editing platform, built on CRISPR/Cas9 technology. It integrates a unique gRNA design algorithm, a comprehensive database of cell line editing parameters, precise pooled editing efficiency assessment, strategies to enhance single-clone formation, and a high-throughput genotyping system suitable for low cell numbers. For generating knockout cell lines, CRISPR-U™ can achieve 10–20 times higher editing efficiency compared with conventional methods.
Q3. What is the difference between gene knockout and knockdown?
Gene knockout cell lines permanently inactivate a target gene, typically via CRISPR/Cas9-mediated deletion, effectively abolishing its function. Gene knockdown cell lines, on the other hand, temporarily or partially reduce gene expression using siRNA, shRNA, or similar approaches, while the gene itself remains intact. Knockout is ideal for in-depth functional studies or disease model development, whereas knockdown is more suitable for preliminary screening or studies involving essential genes. Each method offers distinct advantages in terms of stability, efficiency, and experimental applications.
Ubigene provides not only customized knockout cell line generation but also high-efficiency gene knockdown solutions. Whether your research focuses on functional validation, mechanistic studies, or disease model construction, Ubigene delivers reliable, high-performance gene editing solutions tailored to your needs.
What is the cost of gene knockout (KO) services?
Ubigene offers comprehensive validation data for KO projects. We have already established over 8,000 ready-to-use KO cell lines, with special prices starting from $1980 and delivery as fast as one week. If your target cell line is not available, we also offer customized gene knockout services to meet your specific research needs. Click here to consult online >>>
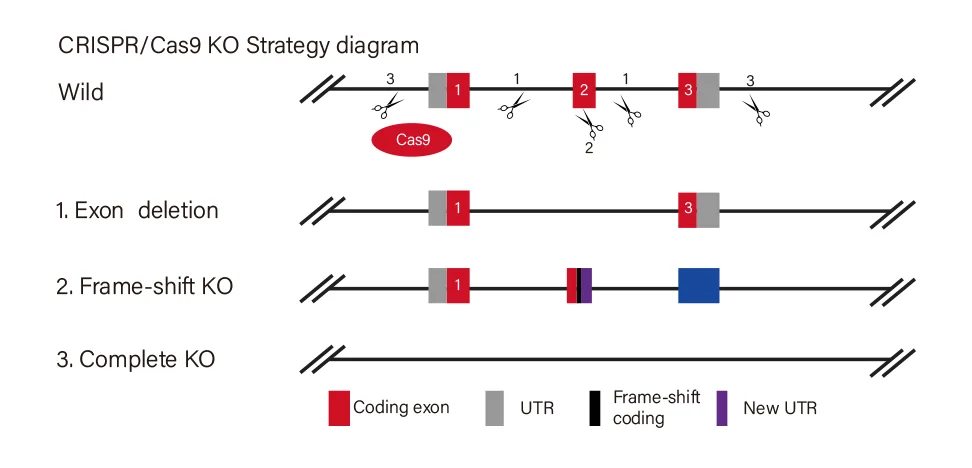
Troubleshooting: Practical Tips
- 1. Low Editing Efficiency: First, assess delivery efficiency using a GFP reporter system. If necessary, increase the MOI (for viral transduction) or switch to an alternative sgRNA.
- 2. Significant Off-Target Effects: Often caused by non-specific Cas9 cleavage or high homology of the sgRNA to other genomic regions. Solutions include using high-fidelity Cas9 variants and shortening the Cas9 expression period.
- 3. High Cell Death Rate: Reduce transfection reagent dosage, adjust the delivery time window, or consider using a conditional knockout strategy.
- 4. Slow Single-cell Clone Growth: Optimize medium composition, supplement growth factors, or use a conditioned incubator.
Conclusion
Gene knockout experiments are a cornerstone technology for dissecting gene function in life sciences. Their success depends on the scientific rigor of the experimental design, the precision of operational details, and the depth of accumulated experimental experience. From the accurate selection of sgRNA targets and matching of the editing system to the cell type, to the optimization of transfection conditions, efficient single-clone screening, and the establishment of multi-dimensional validation systems—every step must be carefully controlled. Any oversight can prolong the experimental timeline, reduce the reliability of results, or even compromise the entire project.
For researchers seeking efficient and robust outcomes, collaborating with a professional team with a mature technological platform can significantly reduce experimental risk. Ubigene has extensive experience in gene editing, leveraging the CRISPR-UTM multi-platform technology matrix, validated standardized workflows (knockout efficiency ≥97%), and expertise in constructing over 10,000 cell models. We provide a one-stop KO solution covering experimental design, cell editing, and positive clone validation. This approach not only helps researchers overcome technical challenges and shorten project timelines but also frees up valuable scientific effort, allowing them to focus on exploring and innovating around core research questions.
Contact us to learn more about our customized KO cell line services >>

