Expert Insights | Hurry up and collect the SH-SY5Y cell tips!


Expert Insights | Hurry Up and Collect the SH-SY5Y Cell Tips

The SH-SY5Y cell line, originating from a bone marrow sample of a 4-year-old girl, is an invaluable tool in human neuroblastoma research. Its significance in neuroscience is paramount, especially for investigating the underlying mechanisms of neurodegenerative diseases like Parkinson's disease. Furthermore, researchers can harness gene editing technologies to unlock the mysteries of specific genes in neurons, shedding light on their crucial roles in various diseases and paving the way for innovative treatments.
When SH-SY5Y cells are cultured and gene-edited, the operating details are very important, small changes may have a certain impact on the accuracy of the experimental results and the stability of the cell state. Today, let us learn more about how to culture SH-SY5Y cells and master the key skills of gene editing!
What Are SH-SY5Y Cells?
Cell Information
|
Cell Name |
SH-SY5Y(Human Neuroblastoma Cell Line) |
|
Cell Morphology |
Epithelial-like, adherent and suspension cells co-exist |
|
Cell Culture Medium |
DMEM+10%FBS+1%P/S |
|
Culture Environment |
Air, 95%; CO2, 5%; 37°C |
|
Medium Change Frequency |
2-3 times a week |
|
Passage Ratio |
1:2-1:3 |
Need other cells for your research? Click to browse our 8000+ complete cell line bank>>>
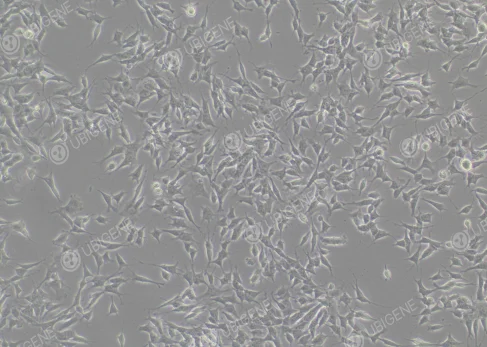

Figure 1. Cell Image (Normal Growth)
(The cells are epithelial-like, most of them grow attached to the wall, and a few are suspended in the culture medium. It is normal for most of the cells to be attached to the wall during the culture process; the attached cells will have short tentacles extending out)

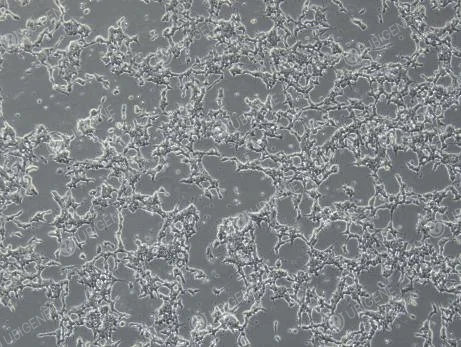
Figure 2. Cell Image (Poor Growth)
(Changes in cell morphology, such as cells becoming rounded, elongated, or losing their tentacles; excessive growth in aggregates; dead cells floating in clumps)
SH-SY5Y Cell Culture Protocol
Cell Thawing
1) Preparation: Take 7mL of complete culture medium and put it in a centrifuge tube for later use.
2) Thawing: Take the cells out of the dry ice, hold the cap with forceps, quickly thaw cells in a 37°C water bath by gently swirling the vial (Note: keep the cap out of the water). In about 1 minute, it would completely thaw.
3) Centrifugation: Transfer the thawed cell suspension to a centrifuge tube, centrifuge at 1100rpm for 4 minutes, and discard the supernatant.
4) Resuspension and inoculation: Resuspend the cells with complete culture medium and inoculate them in a culture plate or culture flask of appropriate size.
5) Culture: Place the culture plate or culture flask in a 37℃, 5% CO2 incubator and observe the cell state and adhesion after 24 hours.
Cell Passaging (Take T25 flask as an example)
1) When the cell confluence reaches 80-90%, it is ready to passage. During passaging, discard the culture medium in the culture flask in the ultra-clean bench, add 5mL PBS to wash the cells 1-2 times.
2) Add 1mL trypsin, gently shake the bottle to make the trypsin completely cover the cells, put the culture flask into the incubator for 1-2 minutes. When most of the cells become round and bright under the microscope, gently shake the sides of the culture flask and then stop digestion immediately when most of the cells fall off.
3) Add 2 times the volume of trypsin, that is, 2mL complete culture medium to stop digestion, and then transfer to a 15mL centrifuge tube.
4) Centrifuge at 1100 rpm for 4 minutes at room temperature. After centrifugation, discard the supernatant and add complete culture medium to resuspend the cells.
5) Cells need to be passaged at a ratio of 1:2-1:3, and observe the cell status the next day.
Cell Cryopreservation
1) Collect cells: Collect digested cells into centrifuge tubes according to the cell passage process.
2) Centrifuge: Centrifuge at 1100rpm for 4 minutes and remove the supernatant.
3) Resuspend and cryopreserve: Resuspend cells with cell cryopreservation solution, distribute into cryopreservation tubes at 1×10^6 cells/mL per 1mL cryopreservation solution, and mark the name, passage number, date etc.
4) Cooling and storage: Place the cryopreservation tubes in a program cooling box, store in a -80℃ refrigerator overnight, and then transfer to a liquid nitrogen tank for storage.
Note:
1. The cells generally grows in mixed morphology of suspension and adherent cells. If most of the cells are adherent and a minority or few of cells are in suspension, the cells can be digested and cultured as adherent cells.
2. If the suspension cells are in the majority during culture, or the cells clump and grow slowly, it is necessary to collect these suspended cells by centrifugation during medium replacement and passaging, and replace with high-quality serum as soon as possible to promote cell adhesion and growth.
3. The cells are sensitive to serum quality, and it is recommended to use high-quality fetal bovine serum for culture.
Download the SH-SY5Y cell culture guide for free>>>
SH-SY5Y Cell Culture - FAQ
Q1. How to deal with the cells in poor cell condition?
1. Culture medium and serum: Make sure to use the correct basal medium and add the appropriate amount of serum. The serum concentration can be adjusted appropriately according to the cell status.
2. Cell culture environment: Confirm whether the culture temperature, humidity, and gas phase conditions are normal.
3. Avoid using expired or long-stored culture medium. It is recommended to use up the fresh complete culture medium within two weeks.
4. Cell passaging operation: When passing cells, pay attention to the digestion time and trypsin concentration to avoid cell damage caused by digesting for too long or too short.
Q2. How to deal with the cell clumping?
1. Adjust cell density
. Control the inoculation density: don't be too high to reduce the chance of cell aggregation. It is recommended to pass the cells at a density of about 80%.
. Low-density passaging: You can try low-density passaging, that is, reducing the number of cells per passaging, which helps the cells to be more evenly distributed in the culture flask.
2. Optimize culture conditions
. Choose medium: Make sure to use the correct medium for SH-SY5Y cell growth and adjust the composition of the medium as needed, such as serum concentration, etc.
. Ensure the culture environment is suitable, such as stable temperature, humidity and CO₂concentration, etc.
3. Pay attention to digestion operation
. Extend digestion time: If cell clumping is caused by insufficient digestion, digesting time can be appropriately extended, but avoid excessive digestion to cause cell damage.
. Gently pipet the cells: During the process of digestion and passaging, gently blow the cell suspension with a pipette to disperse the cell clumps.Pay attention to blowing moderately to avoid bubbles.
If you have more questions, discuss with our experts anytime!
SH-SY5Y Gene Editing Tips
Notes for SH-SY5Y cell transfection
1. Ensure that the cells are in good condition, in the logarithmic growth phase, and the cell density is generally 80-90%.
2. Pay attention to the cell duration of digestion to avoid over-digestion and damage to the cells.
3. During the experiment, the cells should be blown into single cells to avoid cell agglomeration.
4. The cell viability should be ≥80% during the experiment.
Notes for electroporation
1. Control the amount of cells during the electroporation, and inoculate them into a suitable culture dish after electroporation.
2. When electroporating for experiments, ensure that the cell adhesion rate is ≥70%.
3. When electroporating, the experimental time should not be too long.
Notes for the lentiviral transduction:
1. When doing experiments, the cell confluence before transduction must be controlled and should not be too high.
2. Before doing experiments, a preliminary experiment can be conducted to find the most suitable MOI; add the dyeing agent Polybrene before transduction.
3. Before using the transduction reagent, it is necessary to fully mix it to ensure its uniformity.
SH-SY5Y single cloning experiment notes
1. Ensure that the cell state is normal before the cloning experiment. It is recommended to control the cell confluence at around 70%
2. The cell viability during cloning should be ≥ 80%
3. A preliminary experiment can be conducted to find a suitable cloning gradient to avoid a low proportion of monoclones
4. The cell counting after dilution should preferably fall between 1*10^6-2*10^6
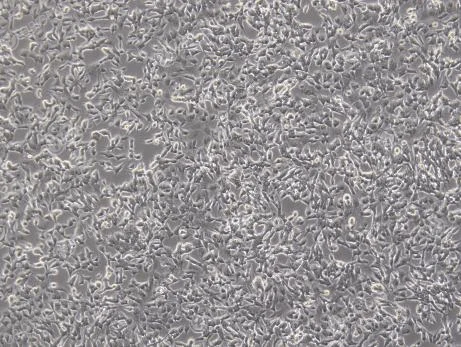

Figure 3. Lentivirus transduction of SH-SY5Y cells
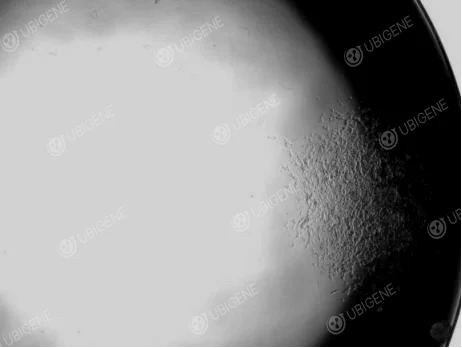
Figure 4. SH-SY5Y cell single clone
Conclusion
Mastering a reliable SH-SY5Y cell culture protocol is essential for ensuring consistent growth, preventing differentiation, and enabling efficient gene editing. From thawing and passaging to lentiviral transduction and single cloning, attention to detail at every step of SH-SY5Y culture can directly influence experimental outcomes—especially in studies of neurodegenerative diseases.
Ubigene provides high-quality SH-SY5Y cells with STR authentication, mycoplasma-free certification, and stable growth performance. Whether you're starting from wild-type cells or planning CRISPR-based gene editing, we're here to support your neuroscience research with robust cell lines and expert editing services.


