1. Development of drug-inducible CRISPR-Cas9 systems for large-scale functional screening
Large-scale genetic screening applying CRISPR/Cas9 technology has become a powerful approach to uncover and validate gene functions. The ability to control the timing of genetic perturbation during CRISPR screens will facilitate precise dissection of dynamic and complex biological processes. It is reported that the optimization of a drug-inducible CRISPR-Cas9 system that allows high-throughput gene interrogation with temporal control.
Researchers have designed multiple drug-inducible sgRNA expression vectors and measured their activities using an EGFP gene disruption assay in 11 human and mouse cell lines. The optimal design allows for tight and inducible control of gene knockout in vitro, and in vivo. Next parallel genome-wide loss-of-function screens were performed using the inducible and constitutive CRISPR-Cas9 systems. In proliferation-based dropout screens, these two approaches have similar performance in discriminating essential and nonessential genes. In a more challenging phenotypic assay that requires cytokine stimulation and cell staining, scientists observed similar sensitivity of the constitutive and drug-induced screening approaches in detecting known hits. Importantly, the minimal leakiness of our inducible CRISPR screening platforms in the absence of chemical inducers in large-scale settings.
2. Conditional gene knockout in human cells with Inducible CRISPR/Cas9
The advent of the easily programmable and efficient CRISPR/Cas9 nuclease system has revolutionized genetic engineering. While conventional gene knockout experiments using CRISPR/Cas9 are very valuable, these are not well suited to study stage-specific gene function in dynamic situations such as development or disease. Here we describe a CRISPR/Cas9-based optimized inducible gene knockout method for conditional loss-of-function studies in human cells. This approach relies on an improved tetracycline-inducible system for conditional expression of single-guide RNAs (sgRNAs) that drive Cas9 activity. To ensure homogeneous and stable expression, the necessary transgenes are expressed following rapid and efficient single-step genetic engineering of the AAVS1 genomic safe harbor. When implemented in human pluripotent stem cells (hPSCs), the approach can be then efficiently applied to virtually any hPSC-derived human cell type at various stages of development or disease.
3. Engineering Human Stem Cell Lines with Inducible Gene Knockout using CRISPR/Cas9
Human pluripotent stem cells (hPSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), are useful tools for elucidating regulatory processes during early development and disease pathogenesis under the human genetic backgrounds. Genetic modification, including gene knockout (KO), further expands the utility of hPSCs in studying gene function in human embryogenesis or human genetic diseases. Thus, precise temporal control of gene KO in hPSCs is often necessary or highly beneficial for elucidating gene functions and molecular pathways that underlie complex human traits. Precise temporal control of gene expression or deletion is critical for elucidating gene function in biological systems. However, the establishment of human pluripotent stem cell (hPSC) lines with inducible gene knockout (iKO) remains challenging. So scientists explored building iKO hPSC lines by combining CRISPR/Cas9-mediated genome editing with the Flp/FRT and Cre/LoxP system and further developed a strategy to simultaneously insert an activity-controllable recombinase-expressing cassette and remove the drug-resistance gene to speeding up the generation of iKO hPSC lines. This two-step strategy was used to establish human embryonic stem cell (hESC) and induced pluripotent stem cell (iPSC) lines with iKO of SOX2, PAX6, OTX2, and AGO2, genes that exhibit diverse structural layout and temporal expression patterns. The availability of iKO hPSC lines will substantially transform the way we examine gene function in human cells.
Gene knockout cutting efficiency can at least give people the ability to generate protein quantification profiles and establish extended records, and has multiple advantages. It is a particularly alternative recombinant protein expression system. Second, the operation is simple, and recombinant protein can be produced quickly by transient gene expression. Third, it can be used for stable recombinant protein production. Some researchers use gene cell knockout cutting efficiency systems to generate genes. Editing the cell line, recombining the GLUL genomic locus, produced a stable cell line of EPO, and found that the recombination contributed to the stable expression of erythropoietin in the human body.
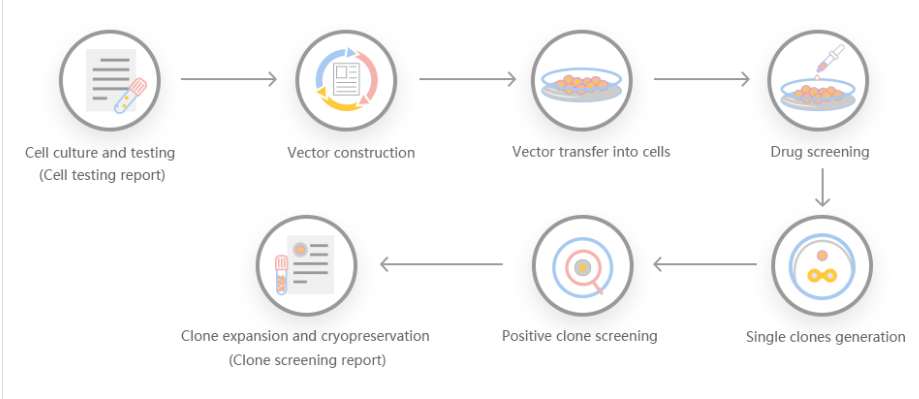
Based on the needs of customers, Yuanjing Biology combined with the situation of target genes to design a stable gene knockout program.
Scheme 1: Small fragment gene knockout scheme. The gRNA is set in the intron of the exon 2 boundary. The number of knockout exon coding sequences is not a multiple of three, which may cause frameshift after knockout.
Scheme 2: Frame-shifting gene knockout scheme, gRNA is set on the exon, the number of missing strings is not a multiple of three, and frame-shift mutation can occur after the knockout.
Scheme 3: Large fragment gene knockout scheme, the entire gene coding sequence is knocked out to achieve the effect of large fragment knockout.
Ubigene Biosciences is co-founded by biological academics and elites from China, the United States, and France. We are located in Guangzhou Science City, which serves as a global center for high technology and innovation. Ubigene Biosciences has 1000㎡ office areas and laboratories, involving genome editing, cell biology technology, and zebrafish research. We provide products and services for plasmids, viruses, cells, and zebrafish. We aim to provide customers with better gene-editing tools for cell or animal research.
We developed CRISPR-U™ and CRISPR-B™(based on CRISPR/Cas9 technology) which is more efficient than general CRISPR/Cas9 in double-strand breaking, CRISPR-U™ and CRISPR-B™ can greatly improve the efficiency of homologous recombination, easily achieve knockout (KO), point mutation (PM) and knockin (KI) in vitro and in vivo.
References:
1) Sun, N., Petiwala, S., Wang, R. et al. Development of drug-inducible CRISPR-Cas9 systems for large-scale functional screening. BMC Genomics 20, 225 (2019).
2) Yuejun Chen, Jingyuan Cao, Man Xiong, Andrew J. Petersen, Yi Dong, Yunlong Tao, Cindy Tzu-Ling Huang, Zhongwei Du, Su-Chun Zhang. Engineering Human Stem Cell Lines with Inducible Gene Knockout using CRISPR/Cas9. Cell Stem Cell. Volume 17, Issue 2, 2015. Pages. 233-244. ISSN 1934-5909,
3) Snijders K.E., Cooper J.D., Vallier L., Bertero A. (2019) Conditional Gene Knockout in Human Cells with Inducible CRISPR/Cas9. In: Luo Y. (eds) CRISPR Gene Editing. Methods in Molecular Biology, vol 1961. Humana Press, New York, NY
Genome Editing Platform
——Focusing on the Application of CRISPR-U™ and CRISPR-B™ Gene Editing Technology
Cell Biology Platform
——Focusing on primary cell
2. Provides culture strategies and related products for different cell types.3. Provides cell biology-related services such as cell isolation, extraction and validation.
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project








