Lysosome-related Gene KO Cell Bank - We have all you want! | Ubigene

Lysosome is an organelle containing a variety of hydrolytic enzymes in cells. It is the main place for cells to decompose and degrade cellular waste and the waste generated during intracellular processes. It plays a key role in many physiological processes, such as cell metabolism, immune response, signaling and so on. Moreover, a number of studies have confirmed that the occurrence of a variety of human diseases may be related to lysosome-related mechanisms, such as atherosclerosis, neurodegenerative diseases, pancreatitis, autoimmune diseases, lysosomal storage diseases and cancer, etc, so a thorough understanding of these mechanisms will help to study lysosome-targeted therapies.

Figure 1. Structure of lysosome
In order to study the function of lysosome-related genes, CRISPR gene-editing technology is usually needed. The research group of Professor Ming Li from the University of Michigan used CRISPR/Cas9 technology to do a genome-wide screening and screened the gene TMEM251 that can hinder RNF152 degradation by affecting lysosomal function[1]; Weber et al performed unbiased metabolic gene screening through CRISPR library and identified two important metabolic pathways related to lysosomal pH environment: cholesterol synthesis and iron uptake[2]. Next, we will take the research recently published on Cell as an example to open up new ideas of lysosomes in Parkinson's disease research with CRISPR gene-editing technology.
[CRISPR/Cas9 gene-editing for TMEM175 - Explore its association with the occurrence of Parkinson's disease]
A research team from Zhejiang University of Technology published an article on Cell entitled “Parkinson’s Disease-Risk Protein TMEM175 is a Proton-Activated Proton Channel in Lysosomes”. This study found that the risk gene TMEM175 for Parkinson’s Disease acts as a proton-activated proton channel on the lysosomal membrane, and its mutation may be one of the key factors to induce Parkinson's disease[3].
TMEM175 was identified as a K+ channel that mediates potassium conductance on lysosomal and endosome membranes, thus regulates the membrane potential and pH of Lysosome in RAW 246.7 mouse macrophage cell line. To determine whether TMEM175 has a similar function in neuron-derived cells, the researchers used CRISPR/Cas9 technology to knock out TMEM175 in SH-SY5Y Neuroblastoma cell line (Figure 2A)[4]. It was found that the lysosome pH of TMEM175 KO SH-SY5Y was slightly higher than that of WT in the medium containing 10% serum. Upon incubation in Earle’s Balanced Salt Solution (EBSS) for 3 hours, the lysosomal pH value increased significantly, while the lysosomal pH of the WT SH-SY5Y cells was maintained in both conditions (Figure 2B). The result is expected given that TMEM175 is generally expressed in multiple tissues in lysosome. Under the fed and starved conditions, the protein levels of lysosomal aspartyl protease, CTSD, and cysteine protease, cathepsin B (CTSB) in TMEM175 KO cells were significantly lower than those of WT (Figure 2D), and lysosome function was impaired. In TMEM175 KO cells, it was also observed that the migration rate of lysosome-associated membrane glycoprotein 1 (LAMP1) was faster than that of WT, which may be caused by deglycosylation or proteolytic cleavage (Figure 2D). TMEM175 KO did not change the number of LAMP1+ lysosomes (Figure 2C). Further evaluation of these lysosomal enzyme activities showed that compared with WT, the CTSD, CTSB and another lysosomal hydrolase GBA were significantly reduced by 20-30% in TMEM175 KO (Figure 2E-G). Starved conditions did not further demonstrate the degree of enzyme activity reduction between WT and KO. Compared with WT, the degradation rate of protein labeled with L-Homopropargylglycine (HPG) in KO decreased (Figure 2H).
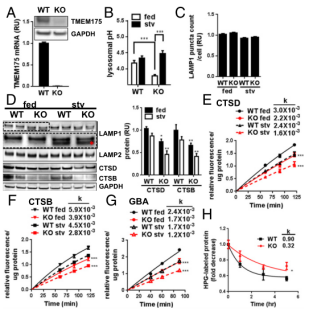
Figure 2. The pH of lysosome in TMEM175 KO cells was unstable and the enzyme activity was reduced
To determine if TMEM175 plays a role in the α-synuclein aggregation, the researchers used shRNA to interfere with TMEM175 in rat hippocampal neuronal cell line, and then assessed the expression of the phosphorylated α-synuclein (Figure 3A) through immunocytochemistry and Western blotting. At 14 and 21 days of culture, the expression level of TMEM175 decreased by 75-80% (Figure 3B). In TMEM175-KD neuronal cells treated with PFF, phosphorylated α-synuclein staining significantly increased 2-3 times (Day 14) (Figure 3C and D), and increased 1.5 times (Day 21) (Figure 3G and H). After 14 days of PFF treatment, there was no change in the cell viability assessed by the number of healthy nuclei or metabolic activity in TMEM175-KD neurons (Figure 3E). 21 days after PFF, there was a decrease in viable cells (Figure 3I), which may lead to attenuated fold-increase in phosphorylated α-synuclein compared with 14 days after PFF. Importantly, compared to the control, the knockdown of TMEM175 led to an increased propensity for accumulation of α-synuclein aggregates, which suggests that it is the key factor in α-synucleinopathies and the development of Parkinson's Disease.
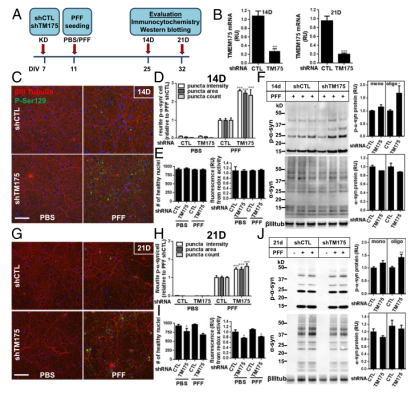
Figure 3. Knockdown of TMEM175 led to an increased propensity for accumulation of α-synuclein aggregates in rat hippocampal neuronal cell line
Researchers investigated the function of TMEM175 in a rat neuronal model system and these studies have confirmed that the lack of TMEM175 leads to the instability of lysosomal pH, which further leads to the reduction of lysosomal catalytic activity, the reduction of glucocerebrosidase activity, impaired autophagosome clearance by the lysosome, and the reduction of mitochondrial respiration. In addition, the loss of TMEM175 in rat primary neurons leads to an increase in susceptibility to exogenous α-synuclein fibrils. After treatment of α-synuclein fibril, it was found that neurons lacking TMEM175 were found to have increased phosphorylated and detergent-insoluble α-synuclein deposits. To sum up, TMEM175 plays a direct key role in lysosomal and mitochondrial functions and the pathogenesis of Parkinson's Disease and highlights this Ion channel as a potential therapeutic target for Parkinson's Disease.
References:
[1] Zhang W, Yang X, Li Y, et al. GCAF (TMEM251) regulates lysosome biogenesis by activating the mannose-6-phosphate pathway[J]. Nature Communications, 2022, 13(1): 5351.
[2] Weber RA et al. Maintaining Iron Homeostasis Is the Key Role of LysosomalAcidity for Cell Proliferation. Molecular Cell. (2020) 7, 645-655.
[3] Hu M, Li P, Wang C, et al. Parkinson’s disease-risk protein TMEM175 is a proton-activated proton channel in lysosomes[J]. Cell, 2022, 185(13): 2292-2308. e20.
[4] Jinna S PE et al. TMEM175 deficiency impairs lysosomal and mitochondrial function and increases α-synuclein aggregation[J]. PANS. (2017)26: 114 (9) 2389-2394.
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project











