IF=16.6 | Development of Non-Small Cell Lung Cancer Drug Therapy Independent of the p53 Signaling Pathway

Due to the high mutation rate of TP53, non-small cell lung cancer (NSCLC) exhibits high drug tolerance, leading to poor treatment efficacy and low survival rates. Cisplatin, as a first-line treatment for NSCLC, also carries the risk of inducing mutations. If p53 mutations accumulate during cisplatin chemotherapy, it will further impact the patient's survival rate after chemotherapy. Therefore, it is crucial to develop a tumor drug therapy solution independent of the p53 pathway.
Recently, a research paper titled “Nanoparticles targeting mutant p53 overcome chemoresistance and tumor recurrence in non-small cell lung cancer” by the research group of Professor Hulin Jiang at China Pharmaceutical University was published on Nature Communications (IF: 16.6). The study used Ubigene's engineered mutant p53 plasmid, and through examining the cisplatin tolerance of plasmid-transfected H1299 cells, thoroughly elaborated the necessity to improve the limitations of cisplatin clinical applications as a starting point from a p53 mutation standpoint.
In this study, the researchers first validated the possible
mechanisms of cisplatin progressive tolerance during tumor therapy, including increased TP53 mutation
risk, which in turn increases cisplatin tolerance. To address this issue, the researchers synthesized
Fluplatin, a prodrug composed of cisplatin and fluvastatin, and constructed the final formulation, FP
NPs. FP NPs, not restricted by p53 mutations, target the endoplasmic reticulum to degrade p53 mutant and effectively
induce endoplasmic reticulum stress independently of the p53 pathway, ultimately exerting effective anti-tumor
effects. FP NPs slow down tumor progression and show the potential for a significant improvement in the adverse
prognosis caused by p53 mutations.
Case study with figures
In this work, the authors validated some possible mechanisms of cisplatin progressive tolerance in non-small cell lung cancer therapy. The anti-tumor effect of cisplatin is associated with an increased TP53 mutation, leading to cisplatin tolerance. The combination of fluvastatin can alleviate the bottleneck of cisplatin therapy (Figure 1).
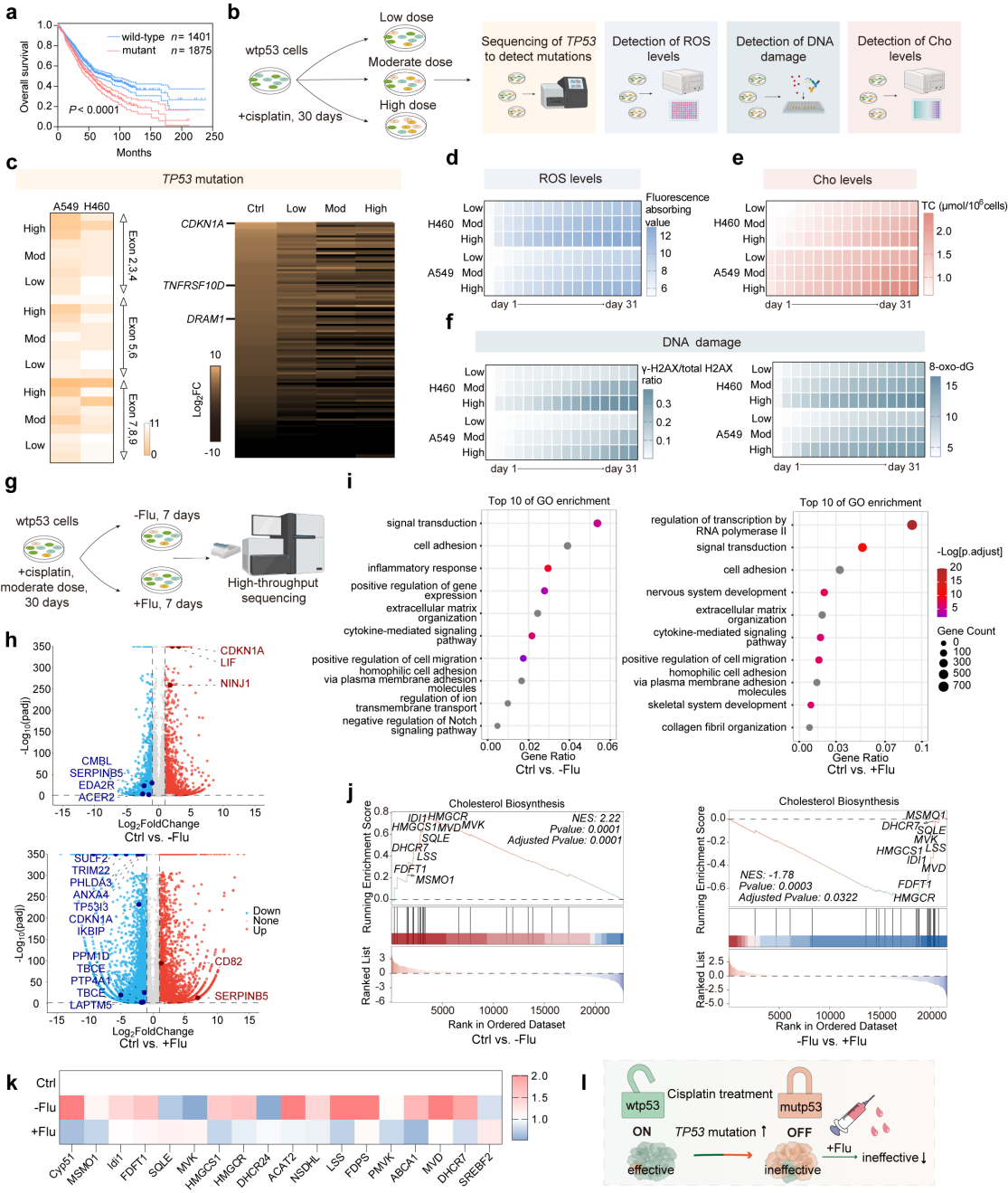
Figure 1. Mechanism
validation of malignant accumulation between cisplatin and p53.
The authors synthesized a prodrug, Fluplatin, composed of cisplatin and fluvastatin through a coordination reaction
and obtained the final formulation, FP NPs. Subsequently, FITC-Dil@FP NPs were constructed, and their
distribution in cells was observed using CLSM. The results are shown in the figures. When Dil entered the cells,
PEG-PE inserted into the cell membrane, and significant separation was observed after 2 hours. Finally, the platinum
content in each organelle of the cells was quantified using ICP-MS. FP NPs were mainly distributed in the
endoplasmic reticulum, and the Pt content in each organelle was significantly higher than that of cisplatin or
Fluplatin.
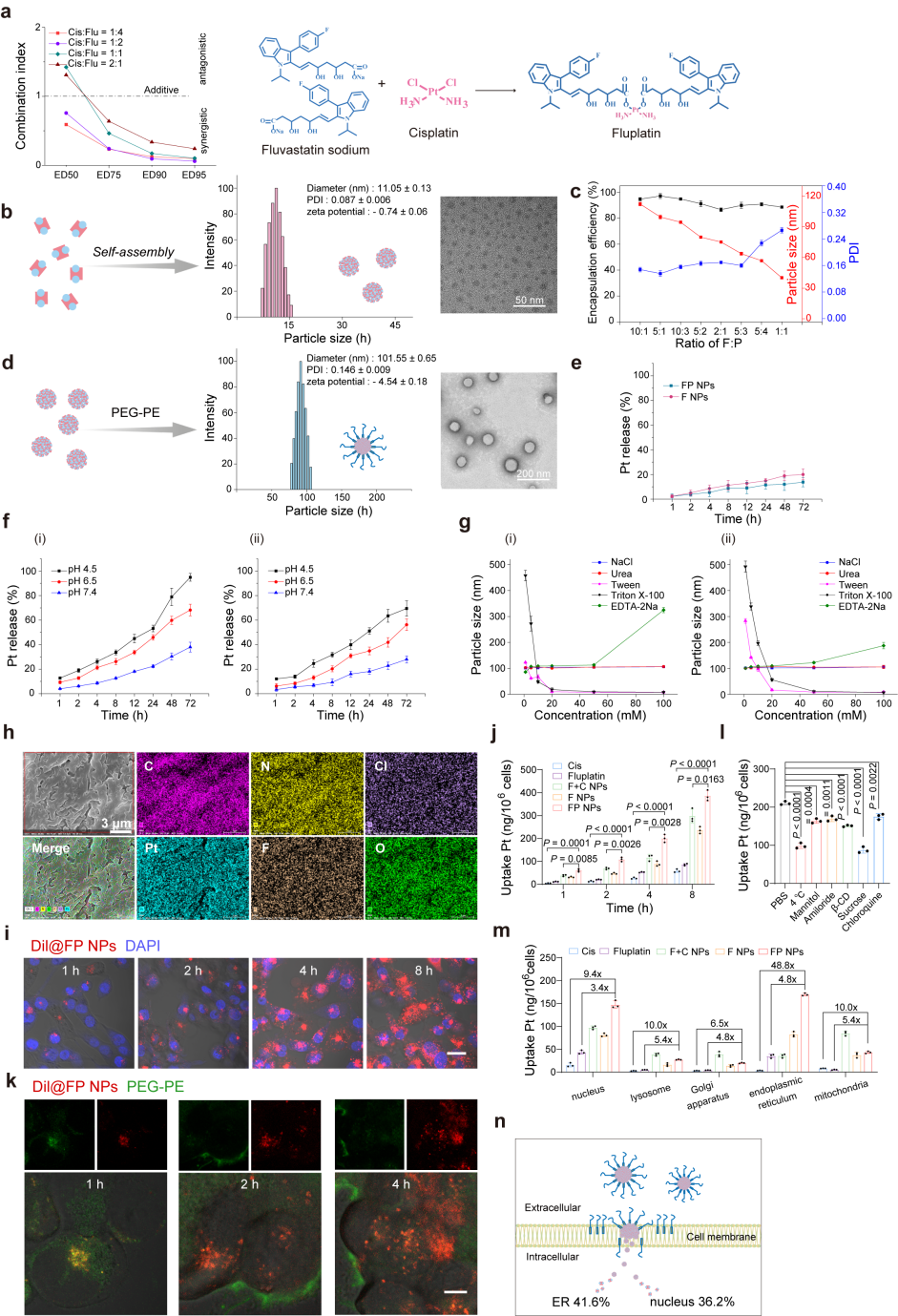
Figure 2. Design
and characterization of FP NPs.
Due to the ER targeting
capability of FP NPs, the researchers studied their induction capability of endoplasmic reticulum stress and
relevant mechanisms. Firstly, they performed co-localization analysis of ER-Tracker Green using CLSM (Figure 3). The
results indicate that Dil@FP NPs continuously accumulated on the endoplasmic reticulum, and the
mitochondrial morphology gradually changed from filamentous to spherical. Consistent with
previously obtained results, significant accumulation of Dil@FP NP in the mitochondria was observed only after 8
hours of Dil@FP NPs treatment, indicating that mitochondrial damage was caused by endoplasmic reticulum
stress. Furthermore, the researchers used JC-1 to check the mitochondrial membrane potential, showing that the
membrane potential induced by FP NPs was the lowest.
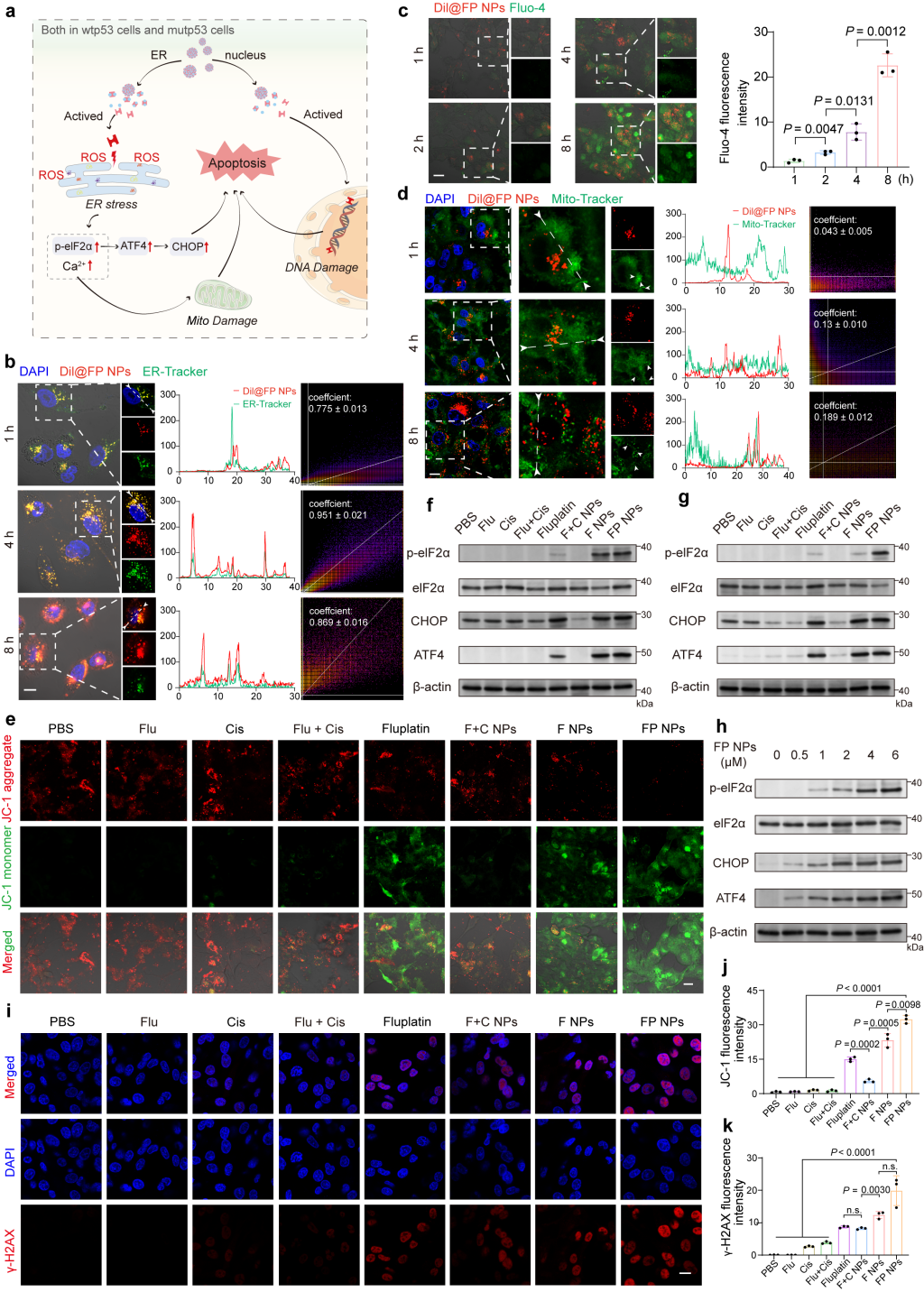
Figure 3. Study of the mechanism of action of FP NPs
Ubigene's newly upgraded CRISPR gene editing system, with over 5000 successful gene editing cases, achieves intelligent customization of gRNA, reducing your trial and error costs, and allowing you to obtain desired data ahead of others! Feel free to contact
us now for gene editing services!
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project