The cell line consists of a population of transformed cells with unlimited division capacity. This is usually due to immortality in the laboratory or due to the tumorigenic origin of the cell line derived from the patient or animal. Cell lines may be invaluable in research and have led to many important discoveries throughout the medical field. They are usually sturdy and require relatively simple conditions and tissue culture. In this way, these types of cells are optimal for knockout cell line technology for proof-of-concept work (such as the development and initial implementation of bioprinting equipment and technology). However, although cell lines do retain some of the normal functions of the cell types from which they are derived, this function is usually greatly reduced.
A cell line containing glutamine synthetase (GS) gene knockout and a method for producing target protein using GS knockout HEK293 cell line
The present invention relates to a novel GS (glutamine synthetase) gene knocked out of the transgenic HEK293 (human embryonic kidney 293) cell line and a method for preparing a target protein using the transgenic HEK293 cell line. In particular, the present inventors eliminated the expression of GS in HEK293 cells to overcome the barriers to cell line selection caused by overexpression of GS, thereby generating target proteins through the GS/MSX system, thereby improving efficiency. Therefore, the selection of cell lines for high-yield target protein will increase, and therefore the protein yield of the selected cell line will increase, which indicates that the gene editing technology of Stable cell line can be effectively used to produce the target protein.
Genetically manipulated cell line reticular cells can dissect the requirements of host malaria invasion
Research on the role of host erythrocyte proteins in malaria infection is hindered by the genetic intractability of this kind of non-nucleated cells. Researchers report that reticulate cells differentiated from a competent immortalized red blood cell line (BEL-A) isolated in vitro support the successful invasion and intracellular development of Plasmodium falciparum. Using CRISPR-mediated gene knockout and subsequent complementary effects, the researchers verified the important role of the red blood cell receptor sinapine in the invasion of Plasmodium falciparum, and proved the rescue of invasive sensitivity through receptor re-expression. The successful invasion of reticulocytes complements the truncated mutants, eliminating the functional role of the basigin cytoplasmic domain during the invasion. In contrast, it is reported that the knockout of cyclophilin B, which is involved in invasion and interacts with basigin, does not affect the invasion sensitivity of reticulocytes. These data establish the use of immortalized red blood cell-derived reticulocytes as a powerful model system to explore hypotheses about the host receptor requirements for Plasmodium falciparum invasion. The researchers showed that reticulocytes derived from immortalized red blood cells support the invasion and development of Plasmodium falciparum, and used CRISPR-mediated gene knockout and complementation of invasion receptors to prove the utility of this model system in malaria invasion research.
A CRISPR/Cas9 genome editing pipeline in the EndoC-βH1 cell line to study genes implicated in beta cell function
Type 2 diabetes (T2D) is a global pandemic with a strong genetic component, but most causal genes influencing the disease risk remain unknown. It is clear, however, that the pancreatic beta cell is central to T2D pathogenesis. In vitro gene-knockout (KO) models to study T2D risk genes have so far focused on rodent beta cells. However, there are important structural and functional differences between rodent and human beta cell lines. With that in mind, researchers have developed a robust pipeline to create a stable CRISPR/Cas9 KO in an authentic human beta cell line (EndoC-βH1). The KO pipeline consists of a dual lentiviral sgRNA strategy and researchers targeted three genes ( INS, IDE, PAM) as a proof of concept. Researcher achieved a significant reduction in mRNA levels and complete protein depletion of all target genes. Using this dual sgRNA strategy, up to 94 kb DNA were cut out of the target genes and the editing efficiency of each sgRNA exceeded >87.5%. Sequencing of off-targets showed no unspecific editing. Most importantly, the pipeline did not affect the glucose-responsive insulin secretion of the cells. Interestingly, comparison of KO cell lines for NEUROD1 and SLC30A8 with siRNA-mediated knockdown (KD) approaches demonstrate phenotypic differences. NEUROD1-KO cells were not viable and displayed elevated markers for ER stress and apoptosis. NEUROD1-KD, however, only had a modest elevation, by 34%, in the pro-apoptotic transcription factor CHOP and a gene expression profile indicative of chronic ER stress without evidence of elevated cell death. On the other hand, SLC30A8-KO cells demonstrated no reduction in K ATP channel gene expression in contrast to siRNA silencing. Overall, this strategy to efficiently create stable KO in the human beta cell line EndoC-βH1 will allow for a better understanding of genes involved in beta cell dysfunction, their underlying functional mechanisms and T2D pathogenesis.
CRISPR-U™ (based on CRISPR/Cas9 technology), developed by Ubigene, is more efficient than general CRISPR/Cas9 in double-strand breaking, and CRISPR-U™ can greatly improve the efficiency of homologous recombination, easily achieve knockout (KO), point mutation (PM) and knockin (KI) in vitro and in vivo. With CRISPR-U, Ubigene has successfully edit genes on more than 100 cell lines.
Reference
Gyun Min Lee, Da Young Yu, Soo Min Noh.Cell line containing a knockout of the glutamine synthetase (GS) gene and a method of producing target proteins using a GS knockout HEK293 cell line.2016.US Patent
Satchwell T J, Wright K E, Haydnsmith K L, et al. Genetic manipulation of cell line derived reticulocytes enables dissection of host malaria invasion requirements[J]. Nature Communications, 2019, 10(1): 3806-3806.
Grotz AK, Abaitua F, Navarro-Guerrero E, Hastoy B, Ebner D, Gloyn AL. A CRISPR/Cas9 genome editing pipeline in the EndoC-βH1 cell line to study genes implicated in beta cell function. Wellcome Open Res. 2020;4:150. Published 2020 Apr 29. doi:10.12688/wellcomeopenres.15447.2.
The efficiency of gene knock-out and cleavage can not only give people the ability to generate protein radical profiles and establish regulatory records, but also has many advantages, making it a particularly attractive recombinant protein expression system. First, it is carboxylated on glutamic acid and sulfated on tyrosine. Second, the operation is simple, and the recombinant protein can be quickly produced through transient gene expression. Third, it can be used for stable recombinant protein production. Some researchers used gene cell knockout and cutting efficiency systems to generate gene-edited cell lines, targeted sequencing of GLUL genomic loci, produced stable EPO cell lines, and discovered the mechanism of stable expression of recombinant erythropoietin in humans .
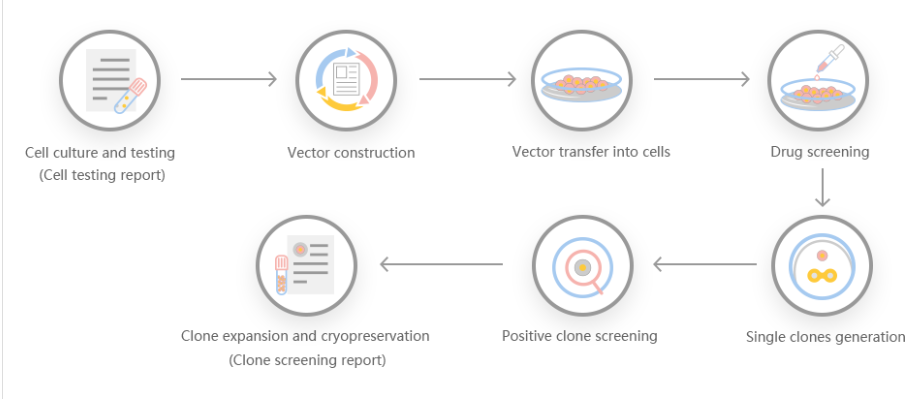
According to customer needs, Yuanjing Biotechnology designs a stable gene transfer knockout program based on the target gene.
Scheme 1: Small-segment gene knockout program, gRNA is set in the introns at both ends of exon 2, and the number of bases encoded by the knockout exon is not 3 times, and the knockout can cause frameshift.
Scheme 2: Frameshift gene knockout scheme, gRNA is set on the exon, the number of missing bases is not 3 times, and frameshift mutation can occur after knockout.
Scheme 3: Large-segment gene knockout scheme, knock out the coding sequence of the entire gene to achieve the effect of large-segment knockout.
Ubigene Biosciences is co-founded by biological academics and elites from China, the United States, and France. We are located in Guangzhou Science City, which serves as a global center for high technology and innovation. Ubigene Biosciences has 1000㎡ office areas and laboratories, involving genome editing, cell biology technology, and zebrafish research. We provide products and services for plasmids, viruses, cells, and zebrafish. We aim to provide customers with better gene-editing tools for cell or animal research.
We developed CRISPR-U™ and CRISPR-B™(based on CRISPR/Cas9 technology) which is more efficient than general CRISPR/Cas9 in double-strand breaking, CRISPR-U™ and CRISPR-B™ can greatly improve the efficiency of homologous recombination, easily achieve knockout (KO), point mutation (PM) and knockin (KI) in vitro and in vivo.
Genome Editing Platform
——Focusing on the Application of CRISPR-U™ and CRISPR-B™ Gene Editing Technology
1. Provides various types of gene-editing vectors for different species.
2. Provides different virus packaging services, including lentiviruses, adenoviruses and adeno-associated viruses.3. Provides high-quality services for gene knockout, point mutation and knockin cell lines.
Cell Biology Platform
——Focusing on primary cell
1. Provides over 400 types of primary cells.
2. Provides culture strategies and related products for different cell types.3. Provides cell biology-related services such as cell isolation, extraction and validation.
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project








