New idea for pancreatic cancer therapy - targeting USP8 enhances PD-L1 immunotherapy sensitivity!
PD-L1, one ligand for Programmed death receptor 1 (PD-1), is a key voucher for T cell to recognize “enemy” and “our side”. Normally, T cells avoid killing their tissue cells by recognizing PD-L1 on tissue cells via surface PD-1 receptors, but some tumour cells also have PD-L1 present on their surface - they can evade immune cell attack with the help of this' green card ', which is also known as immune evasion.
To stop immune escape, the most straightforward way would be to block the combination of PD-1 and PD-L1. This type of inhibition strategy with broad-spectrum antitumor properties has been approved in immunotherapy of many types of cancer, such as breast cancer, melanoma, and non-small cell lung cancer, and the use of this type of immunosuppressant has substantially improved the 5-year survival rate of patients. However, the tumor microenvironment of pancreatic ductal carcinoma is very immunosuppressive, PD-1/PD-L1 blockade is not very effective for pancreatic ductal carcinoma.
Recently, Xueli Bai and Tingbo Liang from Zhejiang University, China found that targeting ubiquitin specific protease 8 (USP8) can increase the sensitivity of pancreatic cancer to PD-L1 immunotherapy, and the combination of USP8 inhibitor and PD-L1 inhibitor can effectively inhibit pancreatic tumor growth, and published at Cell Death & Differentiation as a research article entitled “Targeting ubiquitin-specific protease 8 sensitizes anti-programmed death-ligand 1 immunotherapy of pancreatic cancer”, which provide a new idea for pancreatic cancer therapy.

PD-L1 is regulated by the ubiquitin/proteasome pathway mediated by E3 ligases and deubiquitinating enzymes, suggesting that PD-L1 ubiquitination may represent a breakthrough to reduce tumor immunosuppression. This team had previously demonstrated that the inhibition of the PD-L1 degradation mediated by ubiquitin/proteasome pathway would enhance the immune evasion ability of cancer cells, and found that USP22 could increase the stability of PD-L1 by deubiquitination and thus help the immune evasion of liver cancer cells. These suggest that targeting deubiquitinating enzymes may inhibit the immune evasion of cancer cells.
Like USP22, USP8 is also a deubiquitinating enzyme that catalyzes the release of ubiquitin molecules to protect proteins from degradation and has important roles in cell proliferation, cell cycle, protein surface localization, etc. And there are many studies showing that the mutation or upregulation of USP8 can lead to carcinogenesis, tumor migration, etc. Moreover, USP8 also exerts effects on multiple signaling pathways in many types of tumors, including lung, gastric, breast cancers, etc, which contributes to poor survival of patiens. A role for USP8 in pancreatic cancer has not been reported previously, so Xueli Bai and Tingbo Liang, et al. performed bioinformatics analysis and the analysis showed that USP8 was highly expressed in pancreatic cancer tissues compared to normal tissues (Figure 1b), and a significant correlation between USP8 expression and tumor-node-metastasis stage was observed in clinical pancreatic cancer patients, suggesting that USP8 may be a potential antitumor immunotherapeutic target for pancreatic cancer.
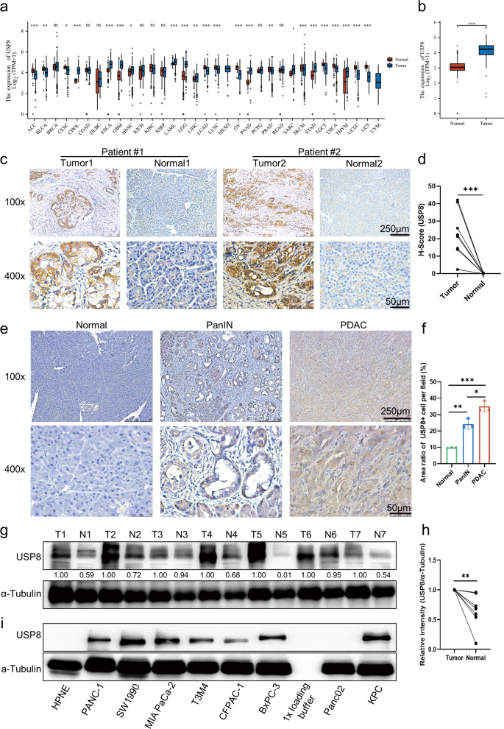
Figure 1. USP8 is highly expressed in tumor tissues in pancreatic cancer
Further studies found that the expression of USP8 was positively correlated with multiple known immunosuppressive factors. The researchers used a USP8 knockdown stable KPC cell line for mouse orthotopic tumorigenesis and found that the tumors formed by USP8 knockdown KPC cell line were milder and smaller than tumors formed by parental KPC cells in immunocompetent mice (Figure 2a). And the survival in immunocompetent mices with USP8 knockdown cell-derived tumors is longer than the mices injected with parental KPC cells. These phenomena were absent in immunodeficient mice, indicating that the antitumor effects exerted by USP8 deletion are immune dependent.
To assess the effect of USP8 inhibition on pancreatic cancer tumorigenesis, the mices were injected subcutaneously with pancreatic cancer cells pretreated with DMSO or USP8-specific inhibitor, and found that USP8 inhibitor pretreatment reduced tumorigenesis in immunocompetent mices (Figure 2l). Besides, the researchers administrated the USP8 inhibitor to mice bearing the constructed pancreatic cancer tumor model and found that the tumor size, volume, and weight were significantly reduced in the USP8 inhibitor group. These data indicate that USP8 is associated with pancreatic tumor development, migration and invasion and is a potential anti-tumor immunotherapeutic target.
Ubigene can provide off-shelf USP8 KO cell lines, customized USP8 KO cell line and customized USP8 knockdown cell line services. The turnaround time for off-shelf cell lines is as fast as 1 week while fast as 4 weeks for custom cell lines, delivering full-allelic knockout clones. Contact us now >>
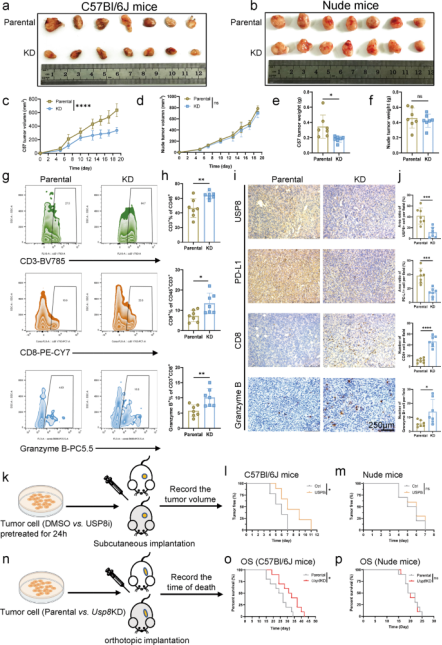
Figure 2. Deficiency of USP8 significantly increases anti-tumor immunogenicity
Furthermore, the researchers also found that USP8 expression was positively correlated with PD-L1 expression in pancreatic cancer tissues, and that USP8 regulated PD-L1 at the post-translational level by regulating its ubiquitination levels, this result was also verified by the application of USP8 inhibitor - which can significantly reduce PD-L1 levels. In vitro and in vivo experimental results demonstrated that USP8 stabilized PD-L1 levels in pancreatic cancer cells by inhibiting PD-L1 degradation mediated by ubiquitin/proteasome pathway, which also implied that targeting USP8 could suppress pancreatic cancer cell immune escape.
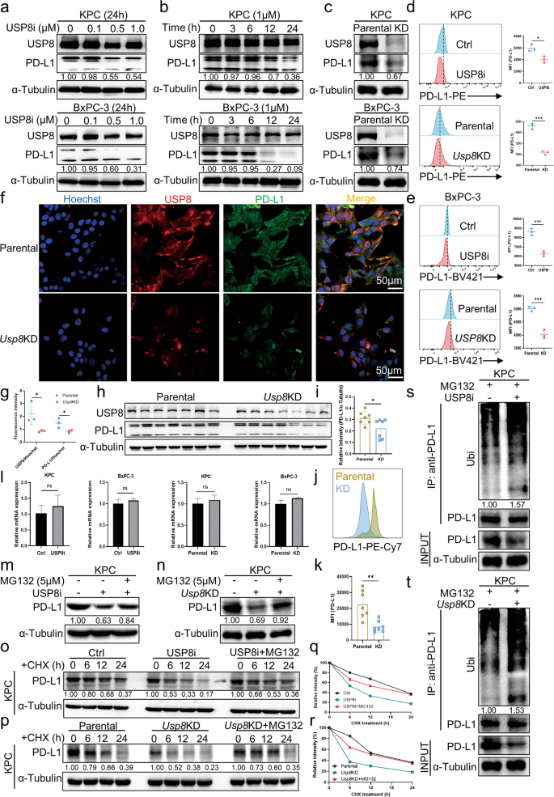
Figure 3. USP8 inhibits ubiquitination-mediated degradation of PD-L1 by proteasomes
The researchers used a combination of USP8 inhibitor and PD-L1 blockade in orthotopic tumorigenic mice and found that the combination activated cytotoxic T cells, enhanced anti-tumor immunity in mice, and effectively inhibited tumor growth and migration. Cellular and mouse level studies using a CRISPR/Cas9-mediated Cd274 knockout KPC cell line revealed that this combined therapy that elicits anti-tumor immunity is dependent on the PD-L1 pathway and CD8 + T cells.
This study revealed the relationship and role of USP8 and PD-L1 in pancreatic cancer, found that the combination of USP8 inhibitor and PD-L1 blockade can effectively inhibit tumor growth, and proposed that USP8 is not only a potential target for immunotherapy of pancreatic cancer but also a key to improve the efficacy of PD-L1 blockade therapy, providing a new idea for immunotherapy strategies for pancreatic cancer.
Ubigene has experience of gene-editing with over 5000 successful cases and has successfully developed CRISPR-U™ technology with 10-fold editing efficiency. Ubigene can provide the custom construction services for the aforementioned knockout and knockdown cell lines, delivery as fast as 4 weeks, price starts from $2980 delivering positive clones. Contact us now >>
Relevant recommendations:
3000+ off-shelf KO cell lines >> From $1980, deliver fast as 1 week, cover thousands of genes in three major modules: signaling pathways, disease models, and drug targets!
10000+ off-shelf gRNA plasmids with high cutting eff iciency[50% OFF] >> Paired with Cas9 stable cell lines to enable high efficient gene knockout!
100+ types of Cas9 stable cell lines >> High stable expression of Cas9, expression report provided!
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project












