Easily explore inflammatory/immune mechanisms with overexpression THP-1 stable cell line

THP-1 cell line is a human monocytic leukemia cell line, which was established in 1980 from the peripheral blood of an acute monocytic leukemia patient, and it is a suspension cell. Compared with U937, HL-60, ML-2, and other leukemia cell lines, THP-1 has morphological and functional characteristics more similar to the human primary monocytes (including cell differentiation markers); Compared with human peripheral blood mononuclear cells (PBMCs), THP-1 is easier to culture and expand in the laboratory, and has a more stable gene background, which can be used in transfection or infection experiments, and is conducive to the reproduction of experimental results. Therefore, THP-1 is an acute monocytic leukemia cell line commonly used in laboratories. By constructing a stable expression THP-1 cell line, it can be used to study the mechanism of immunity and inflammation.
Next, the application of stable expression THP-1 cell lines in the research of inflammation and immunity will be introduced!
Inflammation study on THP-1 macrophage
Astragalus mongholicus polysaccharides (APS) have anti-inflammatory, antioxidant and immunomodulatory effects. Recent studies have found that N6 -methyladenosine (m6A) plays an epigenetic regulatory role in the development of inflammation. However, the effect of APS on m6A is unclear. WTAP is one of the essential proteins of m6A methyltransferase complex and plays an important role in the transcription of m6A. This study explored the mechanism of m6A protein in APS regulation of THP-1 macrophage inflammation by overexpressing WTAP in THP-1 cells [1].
In order to investigate the mechanism of APS regulating m6A modification level, the authors first examined whether APS treatment could affect the expression of the protein related to the m6A modification system in LPS-induced THP-1 macrophages. As shown in Figure 1, the mRNA expression of WTAP was significantly reduced after APS treatment for 24 h, and the protein level of WTAP was also significantly reduced compared with other related proteins, indicating that APS can affect m6A modification level by regulating WTAP expression.
To verify whether APS affects the expression of IL-6 through WTAP, the authors detected the expression of IL-6 mRNA and protein after WTAP overexpression by qRT-PCR and WB, respectively. WTAP overexpression improved the low expression of IL-6 in APS-treated THP-1 macrophages (Figure 2).
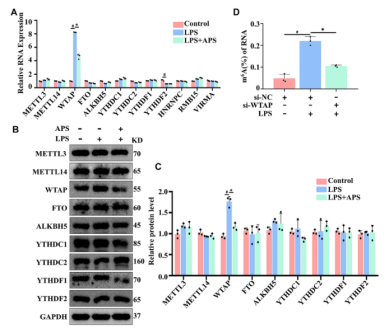
Figure 1. APS reduces WTAP gene expression in LPS-induced THP-1 macrophages
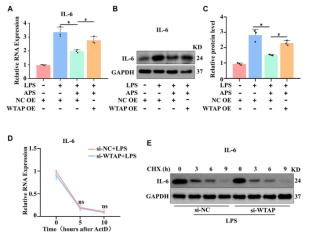
Figure 2. APS exerts anti-inflammatory effects by regulating the expression of WTAP
The authors hypothesized that WTAP regulated IL-6 through the NF-KB pathway, and detected the expression level of p65 in APS-treated THP-1 macrophages by WB. The results showed that APS did not affect the expression of p65 (Figure 3). WTAP overexpression did not affect the overall expression level of p65 in THP-1 macrophages. APS could prevent LPS-induced translocation of p65 from cytoplasm to nucleus in THP-1 macrophages. However, in APS-treated THP-1 macrophages, WTAP overexpression promoted the translocation of p65 from the cytoplasm to the nucleus. In order to better study the nuclear and cytoplasmic distribution of p65, nuclear and cytoplasmic proteins were extracted from THP-1 macrophages. The results showed that APS-mediated inhibition of p65 translocation to the nucleus was reversed in THP-1 macrophages transfected with WTAP overexpression plasmid. These results suggest that APS regulates IL-6 expression through WTAP-mediated p65 nuclear translocation.
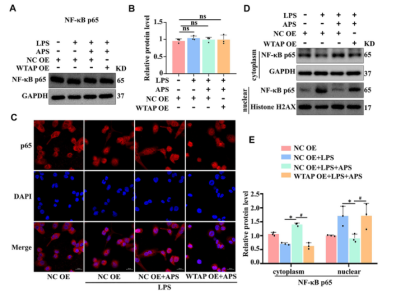
Figure 3. APS regulates IL-6 expression through WTAP-mediated p65 nuclear translocation
In summary, through overexpressing WTAP in THP-1 cells, the authors studied the anti-inflammatory effects of APS mediated by WTAP through regulating m6A modification level in THP-1 macrophages, revealing a new mechanism of APS regulating inflammation at the epigenetic level.
Immune study on THP-1 macrophage
Benzodiazepines are widely used in anesthesia and sedation, and have immunomodulatory properties, but the cellular targets and intermediary signaling pathways involved are still unclear. Therefore, the authors studied the immunomodulatory effect of the benzodiazepine midazolam on human macrophages and its related molecular mechanism [2].
The authors first explored whether midazolam could affect the activation of macrophages through LPS. THP-1 stimulation by LPS could upregulate the expression of the costimulatory molecule CD80 as determined by fluorescence-activated cell sorting and increased the media concentrations of IL-6, TNF-α, IL-10, and NO products (nitrates and nitrites) (Figure 4), while pretreatment with midazolam significantly inhibited the upregulation of CD80 as well as the media concentrations of IL-6, TNF-α, IL-10, nitrates, and nitrites. In addition, the inhibitory effect of midazolam on LPS-induced upregulation of pro-inflammatory molecules was enhanced with increasing dose of midazolam.
The authors pretreated THP-1 cells and PMDMs with various TSPO ligands (RO5-4864, etifoxine and FGIN1-27) and GABAA receptor ligands (vigabatrin and muscimol) before LPS stimulation to investigate whether TSPO receptors contribute to the inhibitory effect of midazolam on macrophage activation. The results showed that TSPO ligands inhibited LPS-induced CD80 upregulation and LPS-enhanced IL-6 release. In contrast, GABAA receptor ligands neither inhibited LPS-induced CD80 upregulation nor IL-6 release. TSPOligands decreased LPS-induced activation of NF-kB/AP-1 in THP-1 Xblue CD14 cells. These results indicated that midazolam reduces the expression of pro-inflammatory factors by activating TSPO signaling, and then blocks the transactivation of NF-kB/AP-1 on related pro-inflammatory genes.
To further investigate whether the inhibitory effect of midazolam on LPS-treated THP-1 cells is dependent on TSPO signaling, the authors constructed a THP-1 cell line with TSPO stable overexpression and compared the dose of midazolam to WT THP-1 cells (Fig. 4). Overexpression of TSPO inhibited the basal expression of CD80 and IL-6, and the inhibitory effect of midazolam on LPS-induced CD80 and IL-6 upregulation was enhanced (that is, midazolam was more sensitive).
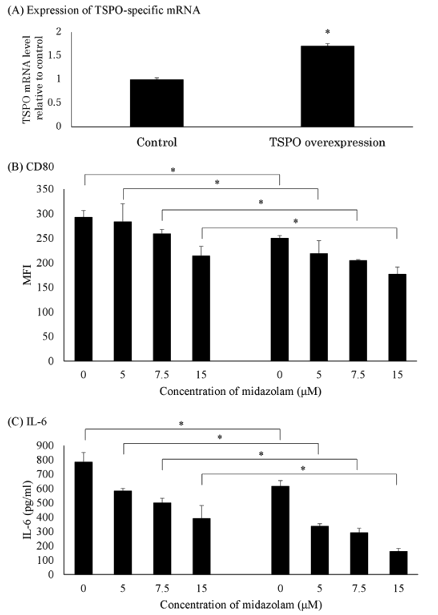
Figure 4. Effect of midazolam on LPS-induced pro-inflammatory response of THP-1 macrophages overexpressing TSPO
The authors observed the effects of midazolam on NF-KB, i -KBα protein and mitogen-activated protein kinase (MAPK) activity in THP-1 cells. The involvement of TSPO ligands in the immunomodulatory effect of midazolam was confirmed by TSPO overexpression, which proved that midazolam inhibits LPS-stimulated immune response in human macrophages by activating TSPO signaling pathway. Inhibition of macrophage activity may lead to deleterious side effects of benzodiazepines in critically ill patients.
References
[1] Long H, Lin H, Zheng P, et al. WTAP mediates the anti-inflammatory effect of Astragalus mongholicus polysaccharide on THP-1 macrophages[J]. Frontiers in Pharmacology, 2022, 13: 1023878.
[2] Horiguchi Y, Ohta N, Yamamoto S, et al. Midazolam suppresses the lipopolysaccharide-stimulated immune responses of human macrophages via translocator protein signaling[J]. International immunopharmacology, 2019, 66: 373-382.
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project












