IF=16.6 |AAV packaged by Ubigene helps construct an arthritis disease model

Rheumatoid arthritis (RA) is a chronic systemic immune disease with unknown etiology. The pathogenesis is still unclear, and the incidence increases with age. The current study shows that a variety of factors including age, smoking history, immunogenicity and genetic polymorphism may be related to the pathogenesis of RA. Metabolic DNA damage and sustained activation of the immune system are important biological activities that promote cellular senescence. During aging, DNA fragments accumulate and circulate in the blood, becoming circulating free DNA (cfDNA), which is also considered as a biomarker of many diseases.
Dr. Neher’s Biophysics Laboratory for Innovative Drug Discovery/State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology (Macau, China) and the Affiliated Hospital of Southwest Medical University, Southwest Medical University (Luzhou, 646000, China) jointly published an article entitled Age-related self-DNA accumulation may accelerate arthritis in rats and in human rheumatoid arthritis on Nature Communications. The authors utilized overexpression AAV packaged by Ubigene to construct a rat model, which confirmed that DNA fragments were inflammatory mediators, and TREX1 gene plays an immunomodulatory role in the pathogenesis of rheumatoid arthritis (RA) [1].
In this study, the authors hypothesized that cellular free DNA (cfDNA)-mediated autoimmune activation and dysfunction of DNA scavenging enzymes may be the causes of RA in older adults. To investigate whether the accumulation of DNA fragments can stimulate abnormal immune responses, the gene expression levels of TREX1 (DNA exonuclease) and cGAS (DNA fragment sensor) in peripheral blood samples of healthy volunteers, RA patients and osteoarthritis (OA) patients were first determined. As shown in Figure 1, the expression level of TREX1 in RA patients was significantly lower than that in healthy controls and OA patients, while the expression level of cGAS was significantly higher, indicating that TREX1 is closely related to the pathogenesis of RA. In the rat model, the transcript levels of TREX1 and cGAS in the blood samples of middle-aged healthy rats were higher than those of young healthy rats. In the peripheral blood serum of healthy children, healthy elderly and elderly RA patients, the serum cfDNA concentration of healthy elderly and elderly RA patients was significantly higher than that of healthy children. In addition, cfDNA accumulation was higher in older RA patients compared with healthy older adults. These findings may indicate that the level of cytoplasmic DNA fragments increases with age, and the accumulation of DNA fragments may activate the cGAS signaling cascade. In addition, the high-level expression of TREX1 is a necessary condition to remove excessive DNA fragments in middle-aged rats, which can prevent abnormal immune responses and maintain the healthy state of animals.
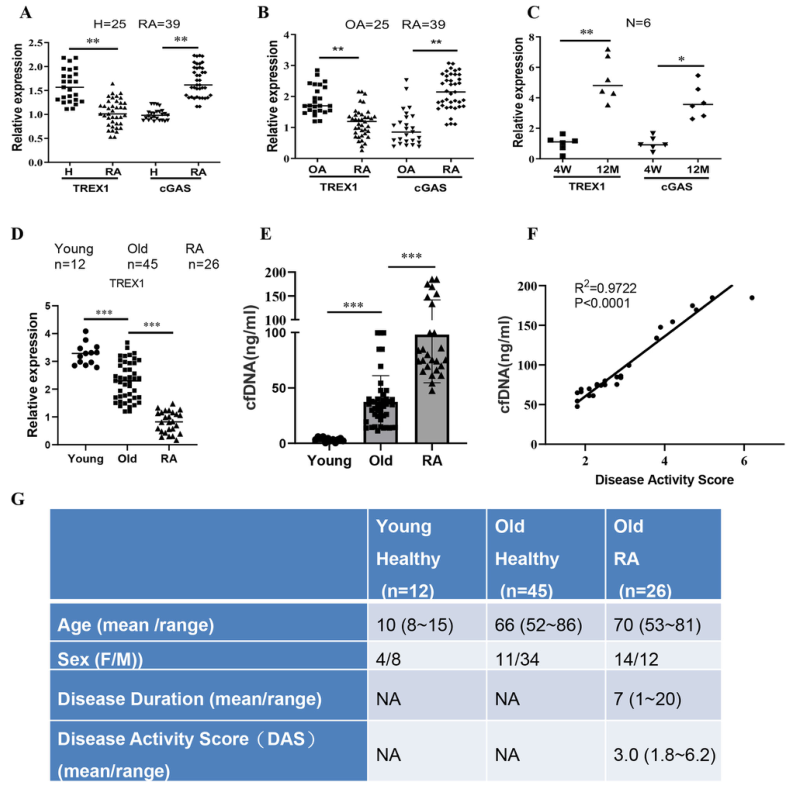
Figure 1. Expression levels and correlation of TREX1 and cGAS in different populations
Subsequently, the inflammatory response of AIA rat model was observed by injecting DNA fragments into the joint cavity and tail vein, and the amount of circulating cfDNA in the body was measured. DNA fragments were injected into the tail vein of healthy rats, and the concentration of serum DNA fragments was measured. The results showed that injection of 200 μg DNA fragment, severe swelling, erythema, joint stiffness and other reactions occurred in the knee joint cavity of AIA rats. On days 9-21, the arthritis condition of AIA rats was more serious than that of non-injected AIA rats, the amount of cfDNA was significantly increased, the gene expression of TREX1 was significantly downregulated, and the cGAS-STING signal regulator and pro-inflammatory cytokine TNF-ɑ, IL-1β, IL-2, IL-6 in the peripheral blood of AIA rats were significantly up-regulated. Micro CT analysis showed that the bone mineral density (BMD), tissue mineral density (TMD), trabecular number and total porosity of swollen joints were low, indicating that the cartilage and bone of AIA rats injected with DNA fragments intravenously were damaged, indicating that the accumulation of DNA fragments may promote bone destruction in AIA rats.
In order to clarify the role of TREX1 in the pathogenic mechanism of DNA fragments in AIA model, cre-loxp-mediated conditional knockout of TREX1 was completed in rats by genome editing mediated by CRISPR technology. TREX1Cre rats were injected with Cre adeno-associated virus 1 week before the establishment of AIA model. As shown in Figure 2, after intra-articular injection of DNA fragments, the soles of TREX1Cre/+ AIA rats and healthy control TREX1Cre/+ rats showed severe foot swelling, erythema and joint stiffness. Compared with AIA rats, the arthritis of AIA TREX1Cre/+ rat models was significantly aggravated. Healthy control TREX1Cre/+ rats successfully induced arthritis by intra-articular injection of DNA fragments. CT analysis further revealed that the bone mineral density (BMD), cortical mineral density (Ct.TMD), bone volume fraction, trabecular number, and total porosity of AIA model TREX1Cre/+ rats injected with DNA fragments and healthy control TREX1Cre/+ rats were severely damaged and showed the most serious joint swelling and cartilage erosion.
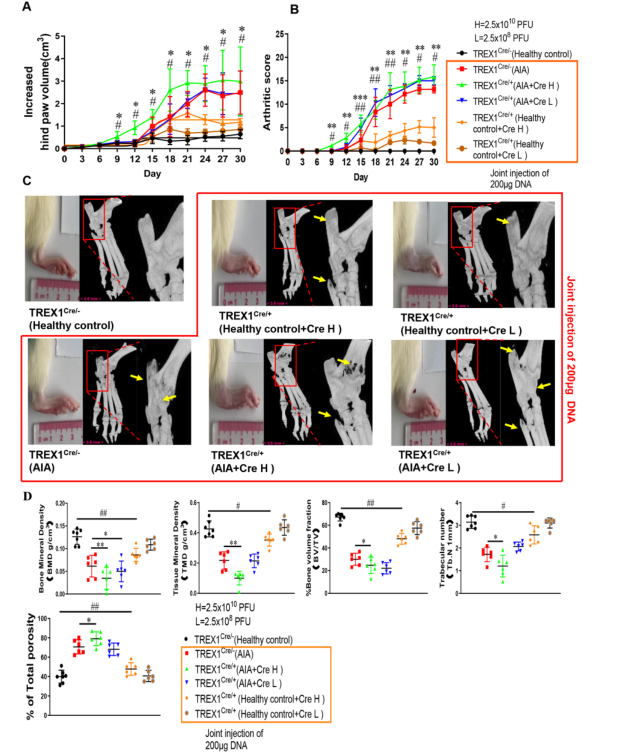
Figure 2. Effect of DNA fragment injection on inflammation in AIA model of TREX1 conditional knockout (TREX1Cre) rats
Next, sonicated rat muscle DNA fragments were injected into both healthy control TREX1Cre/+ rats and AIA model TREX1Cre/+ rats via tail vein to observe the autoimmune response induced by DNA fragment stimulation. The results were consistent with the expected results. The swollen joints and arthritis of healthy control TREX1Cre/+ rats and AIA model TREX1Cre/+ rats injected with DNA fragments were more serious, the bone destruction was serious, the proportion of CD8+ group was significantly increased, and the Foxp3 cell population was significantly reduced.
Further, the adeno-associated virus overexpressing TREX1 (AAV-TREX1) constructed by Ubigene was injected into the joint cavity and tail vein of rats. As shown in Figure 3, AIA rat had severe swelling, erythema and joint stiffness in the hind paw. In contrast, similar to MTX-treated rats, the arthritis score and hind paw volume of AIA rats injected with AAV-TREX1 in the joint cavity or tail vein were significantly reduced, indicating that the severity of AIA in TREX1 overexpressing rats was significantly reduced. AAV-TREX1 injection in the joint cavity or tail vein could significantly reduce the concentration of DNA fragments in the serum of AIA rats, indicating that TREX1 may inhibit joint swelling by scavenging DNA fragments. Taken together, these findings suggest that TREX1 may play an inhibitory role in the development of arthritis in AIA rats via the clearance of DNA fragments.
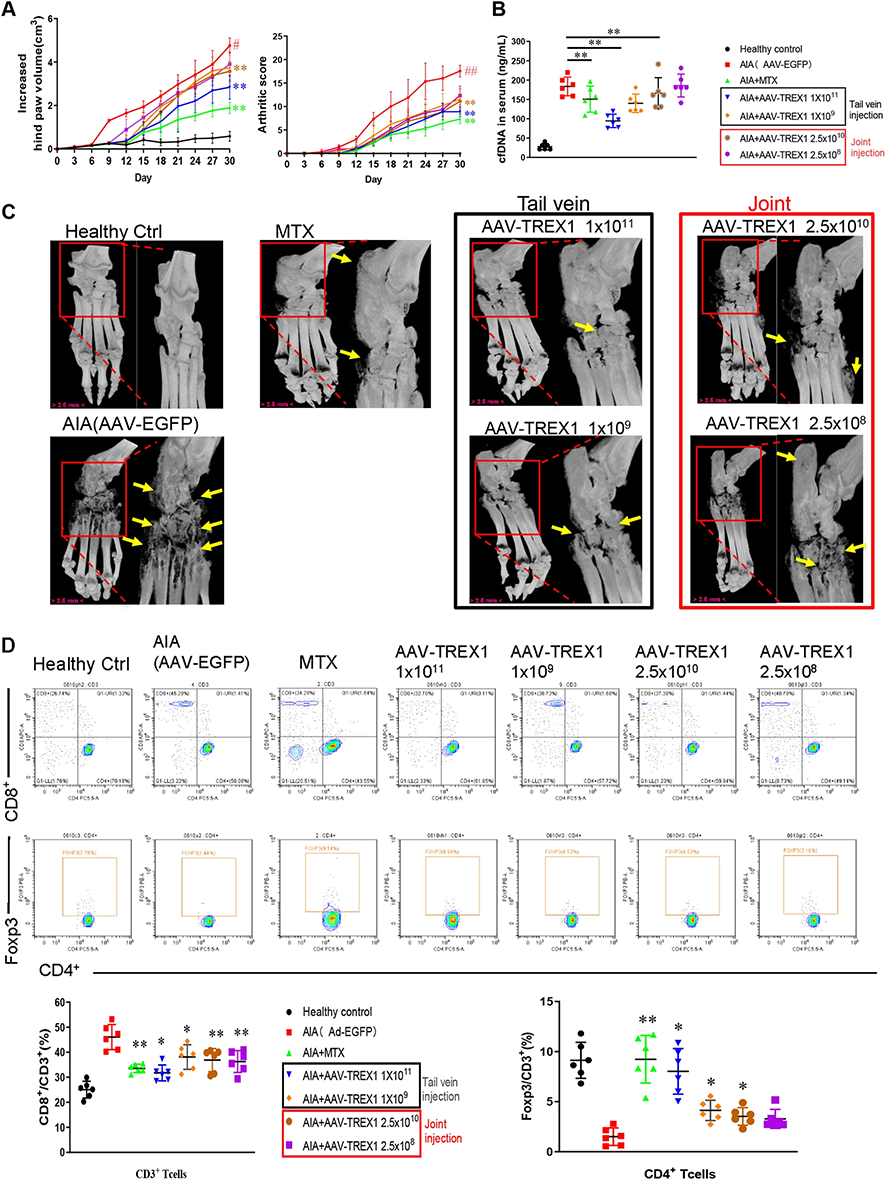
Figure 3. Anti-inflammatory effect of TREX1 on AIA rats
Based on the above in vivo experimental data, SD rats of AIA and TREX1Cre/+ models both showed senescence-associated secretory phenotype (SASP), and after MTX treatment or injection of AAV-TREX1, the arthritis condition and senescence phenotype of these rats were reversed, while the expression levels of pro-inflammatory cytokines and senescence markers such asIL-1β, IL-6, IL-8, IFN-β, p16, and p21 were decreased. Therefore, TREX1 expression may be related to aging at the onset of RA, and TREX1 abnormalities and cfDNA accumulation may be risk factors that promote the progression and development of RA.
Aging is an inevitable process in human beings, which is associated with the pathogenesis of many diseases, including rheumatoid arthritis (RA). It is worth noting that after the age of 50, the immune system has undergone tremendous changes, losing the ability to regulate immunity and gaining proinflammatory functions. Therefore, immunosenescence is particularly accelerated in RA patients, and ultimately promote apoptotic cell death and DNA fragment release.
In this paper, TREX1 overexpressing adeno-associated virus was used to establish an arthritis disease model. It was demonstrated for the first time that DNA fragments may be potential immunogenic factors to induce the development of RA. Elevated cfDNA concentration and decreased TREX1 expression may be one of the clinical manifestations of premature aging. The downregulation of TREX1 may contribute to the accumulation of DNA fragments in RA patients, thereby further aggravating the inflammatory response by activating cGAS/STING signaling. Linking autoimmune tissue inflammation with aging and identifying effective clearance of DNA fragments are important strategies to prevent RA.
Reference
[1] Luo W D, Wang Y P, Lv J, et al. Age-related self-DNA accumulation may accelerate arthritis in rats and in human rheumatoid arthritis[J]. Nature Communications, 2023, 14(1): 4394.
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project











