mTOR may have high research value
in disease treatment and drug development-Then CRISPR/Cas9 can help!
Mammalian target of rapamycin, mTOR is a serine/threonine-protein kinase that is a member of the PIKK protein family and, upon activation by a variety of stimuli, mediates downstream pathways in the form of two complexes, mTORC1 and mTORC2, which regulate various physiological activities including protein synthesis, metabolism, autophagy and cell survival. mTORC1, mentioned above, is composed of mTOR, Raptor, mLST8, Deptor, PRAS40, while mTORC2 is composed of mTOR, Rictor, mLST8, mSin1, PRR5.
The signaling pathways involved upstream and downstream of mTOR are over 30, and the interactive relationship is intricate. Here we simplified a schematic diagram (Figure 1). From Figure 1, there are many factors that activate the mTOR pathway, including cellular nutrition, energy dificit, oxygen levels, mechanical stimuli, amino acids, and hormonal growth factors, etc. These stimulatory factors activate mTORC1 or mTORC2 complexes through upstream signaling pathways (e.g. AMPK, MAPK or PI3K/Akt), which then act on downstream effector molecules. CLIP-170 among downstream effector molecules has been implicated in tubulin assembly, Grb10, Lipin-1 in lipid metabolism, ATG1 in autophagy, 4E-BP, eIF4E, S6K, eIF4B in protein synthesis, Rho and PKC in association with the cytoskeleton, and SGK1 in cell survival.
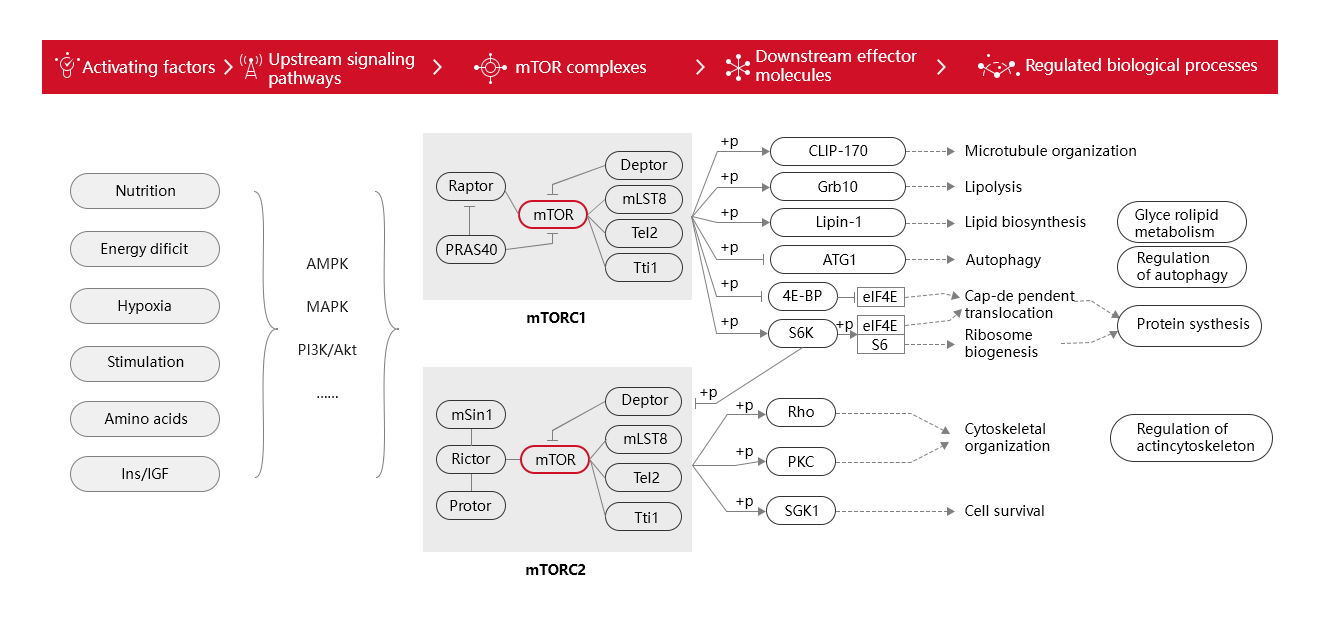
Figure 1. A schematic
diagram of mTOR signaling pathway
Because of this, mTOR can regulate various cellular
physiological activities and participate in the occurrence and development of several human diseases, such as
cardiovascular diseases, glycolipid metabolism disorders, neurological diseases, cancer, and so on. Exploring
the disease-related genes and pathogenic mechanisms in the mTOR pathway can help to identify more potential drug
targets. A few cases of mTOR pathway genes being implicated in disease studies will be presented below.
DEPTOR suppresses tumorigenesis of ESCC cells and
becomes a potential therapeutic target or prognostic marker for human ESCC
As an mTOR binding protein that can inhibit the kinase activities of both mTORC1 and mTORC2, upregulation of DEPTOR expression has antitumor activity in
colorectal, liver, multiple myeloma, and pancreatic cancers. Numerous experiments have shown
that downregulation of DEPTOR expression activated the Akt/mTOR pathway, which directly phosphorylated downstream SGK1 and its substrate
NDRG1, thereby promoted tumor proliferation. Ji et al. [2] found the expression of DEPTOR was significantly decreased in the tumor tissues derived from human ESCC patients and called the
downregulated expression of DEPTOR to be a prognostic marker for ESCC. Moreover, they
also found that DEPTOR expression negatively regulated the tumorigenic activities of ESCC cell lines (KYSE150,
KYSE510 and KYSE190). CRISPR/Cas9-mediated DEPTOR knockout would significantly promote
ESCC cell line proliferation, migration and invasion. Moreover, in
vivo assays further revealed that the growth of KYSE150 cells ectopically
expressing DEPTOR was significantly inhibited compared with untreated KYSE150 cells, whereas the
proliferation, migration and invasion in DEPTOR knockout KYSE150 cells were significantly
enhanced. Most importantly: after biochemical examination, it was found that the phosphorylation of Akt, mTOR
and downstream SGK1 were all downregulated in DEPTOR overexpressed KYSE150, indicating that DEPTOR overexpression can inhibit the Akt/mTOR pathway, thereby controlling ESCC
progression.
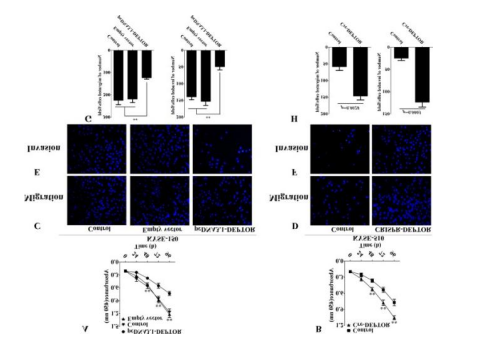
Figure 2. DEPTOR suppressed ESCC cell line
proliferation, migration and invasion
Ubigene can provide
off-shelf DEPTOR knockout cell lines and custom gene overexpression cell line service. Off-shelf KO cell lines
start from $1780, delivery as fast as 1 week
only. Custom gene overexpression cell line services start from $2020, delivering cells with stable expression, good cell
condition. Optional deliverables of stable pools or clones. Click here to
seek exclusive customer services >>
New avenue for
development of mTORC2-specific inhibitor - Distupting the mTOR-mLST8 interaction
It is well known that mLST8 is a shared component of both
mTORC1 and mTORC2 complexes, but little is known about how mLST8 promotes the assembly of mTOR complexes and
maintains their activities. Hwang et al. [3] designed sgRNAs targeting mLST8 exons 2, 4, and 7 to obtain an MLST8 knockout 293T cell line by CRISPR/Cas9. It was experimentally verified
that MLST8 protein knockout affected the assembly of mTORC2 with RICTOR and SIN1 to abolish
mTORC2 activity, but had no effect on mTORC1 activity. Meanwhile, Hwang et al. also constructed mutants of mTOR by homologous recombination repair using CRISPR/Cas9 technology, and identified the interaction sites
of mTOR and MLST8. SIN1 knockout 293T cell line was
generated by gene-edited SIN1 encoding gene MAPKAP1 by CRISPR/Cas9, confirming that mLST8 is the molecular bridge between mTOR and SIN1 in mTORC2. It also suggests
that disrupting the mTOR-mLST8 interaction may be a novel strategy for mTORC2-specific
inhibitor design.
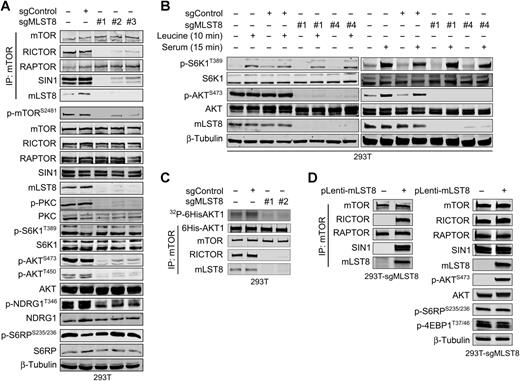
Figure 3. mLST8 was required for mTORC2 assembly
and activity
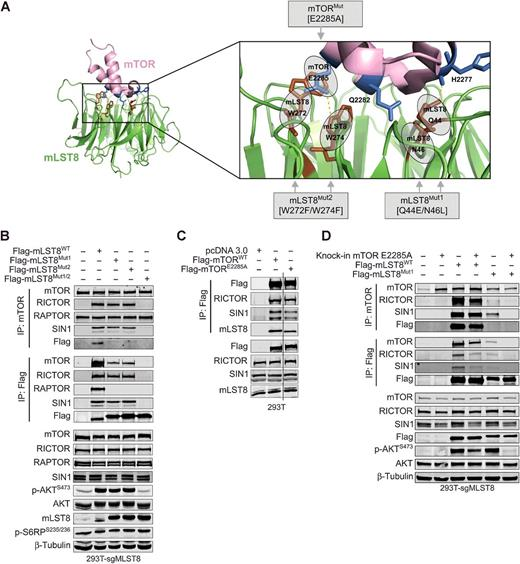
Figure 4. Mutation disrupting the mTOR-mLST8
interaction affected mTORC2 assembly and activity
PRAS40 inhibited
atherogenesis by inhibiting mTORC1-dependent pro-inflammatory signaling in endothelial cells
Activation of pro-inflammatory signals in endothelial cells is
key to atherosclerosis, and many pro-inflammatory and atherogenic signals have focused on the mTOR signaling
pathway. Preclinical studies have found that mTORC1 inhibitors reduced the degree of atherosclerosis but
triggered side effects such as insulin resistance and dyslipidemia, which are quite limited in clinical use.
Zhang et al. [1] selected PRAS40 (AKT1S1), an endogenous regulator gene of mTORC1, for functional validation in
an attempt to find novel gene targets. It has been shown that downregulating PRAS40 expression
in human umbilical vein endothelial cells (HUVECs) can activate TNFα-induced
mTORC1 signaling, promote cell proliferation, upregulate inflammatory markers and monocyte recruitment
levels. By contrast, overexpression of PRAS40 blocked mTORC1 and all proinflammatory
signaling. Furthermore, in an in vivo model of atherogenic remodeling,
endothelium-specific PRAS40 knockout mice showed an enhanced endothelial proinflammatory activation response and
increased neointimal hyperplasia and atherosclerotic lesion formation. These data suggest that PRAS40 suppresses atherosclerosis by inhibiting endothelial mTORC1-mediated pro-inflammatory
signaling. Combined with its favorable effects on metabolic homeostasis, PRAS40 emerges as
a potential target for the treatment of atherosclerosis.
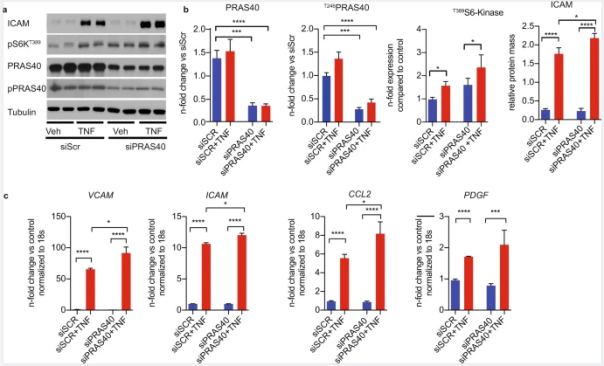
Figure 5. PRAS40 knockdown promoted proinflammatory
signaling in endothelial cells
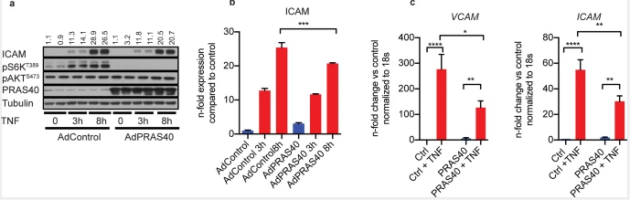
Figure 6. PRAS40 overexpression attenuated
proinflammatory signaling in endothelial cells
mTOR holds great potential for studies of disease processes
and therapeutics, and validation of these mechanisms of action will likely require a large number of knockout or
overexpression cells. Ubigene has recently established mTOR pathway genes knockout cells, KO cell lines for
PRAS40 (AKT1S1), DEPTOR, mLST8, SIN1 (MAPKAP1),
and other mTOR pathway related genes (e.g., DVL1, TSC1, SGK1) noted in the literature are also available in
stock now! Over 3000 KO cell lines as low as $1780, as fast as 1-week delivery! Click here to search for the cell line of your interest from our Cell Line Bank > >
Ubigene also provide speedy customization of KO cells as fast
as 4 weeks, click here for more custom service details
>>
References:
[1]Zhang K S, Schecker J, Krull A, et al. PRAS40 suppresses atherogenesis through inhibition
of mTORC1-dependent pro-inflammatory signaling in endothelial cells[J]. Scientific reports, 2019, 9(1):
1-13.
[2]Ji YM, Zhou XF, Zhang J, Zheng X, Li SB, Wei ZQ, Liu T, Cheng DL, Liu P, Song K, Tan T,
Zhu H, Guo JL. DEPTOR suppresses the progression of esophageal squamous cell carcinoma and predicts poor
prognosis. Oncotarget. 2016 Mar 22;7(12):14188-98. doi: 10.18632/oncotarget.7420. PMID: 26893358; PMCID:
PMC4924707.
[3]Hwang Y, Kim L C, Song W, et al. Disruption of
the Scaffolding Function of mLST8 Selectively Inhibits mTORC2 Assembly and Function and Suppresses
mTORC2-Dependent Tumor Growth In VivoSelective Role of mLST8 in mTORC2[J]. Cancer research, 2019, 79(13):
3178-3184.
 Subscribe Us
Subscribe Us Gene Editing Services
Gene Editing Services
 EZ-editor™
EZ-editor™ Red Cotton Gene knockout Project
Red Cotton Gene knockout Project














